The ER stress transducer IRE1β is required for airway epithelial mucin production
- PMID: 23168839
- PMCID: PMC4031691
- DOI: 10.1038/mi.2012.105
The ER stress transducer IRE1β is required for airway epithelial mucin production
Abstract
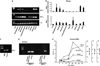
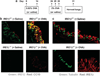
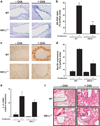
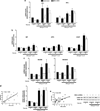
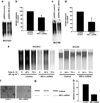
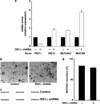
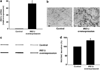
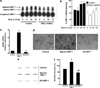
Inflammation of human bronchial epithelia (HBE) activates the endoplasmic reticulum (ER) stress transducer inositol-requiring enzyme 1 (IRE1)α, resulting in IRE1α-mediated cytokine production. Previous studies demonstrated ubiquitous expression of IRE1α and gut-restricted expression of IRE1β. We found that IRE1β is also expressed in HBE, is absent in human alveolar cells, and is upregulated in cystic fibrosis and asthmatic HBE. Studies with Ire1β(-/-) mice and Calu-3 airway epithelia exhibiting IRE1β knockdown or overexpression revealed that IRE1β is expressed in airway mucous cells, is functionally required for airway mucin production, and this function is specific for IRE1β vs. IRE1α. IRE1β-dependent mucin production is mediated, at least in part, by activation of the transcription factor X-box binding protein-1 (XBP-1) and the resulting XBP-1-dependent transcription of anterior gradient homolog 2, a gene implicated in airway and intestinal epithelial mucin production. These novel findings suggest that IRE1β is a potential mucous cell-specific therapeutic _target for airway diseases characterized by mucin overproduction.
Conflict of interest statement
Figures



Similar articles
-
Evolution and function of the epithelial cell-specific ER stress sensor IRE1β.Mucosal Immunol. 2021 Nov;14(6):1235-1246. doi: 10.1038/s41385-021-00412-8. Epub 2021 Jun 1. Mucosal Immunol. 2021. PMID: 34075183 Free PMC article. Review.
-
IRE1α Is a Therapeutic _target for Cystic Fibrosis Airway Inflammation.Int J Mol Sci. 2021 Mar 17;22(6):3063. doi: 10.3390/ijms22063063. Int J Mol Sci. 2021. PMID: 33802742 Free PMC article.
-
T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway.Toxicology. 2019 Aug 1;424:152230. doi: 10.1016/j.tox.2019.06.001. Epub 2019 Jun 4. Toxicology. 2019. PMID: 31170431
-
Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production.J Immunol. 2015 May 1;194(9):4498-506. doi: 10.4049/jimmunol.1401399. Epub 2015 Mar 27. J Immunol. 2015. PMID: 25821218 Free PMC article.
-
IRE1α: from the function to the potential therapeutic _target in atherosclerosis.Mol Cell Biochem. 2024 May;479(5):1079-1092. doi: 10.1007/s11010-023-04780-6. Epub 2023 Jun 13. Mol Cell Biochem. 2024. PMID: 37310588 Review.
Cited by
-
Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease.Gastroenterol Res Pract. 2015;2015:328791. doi: 10.1155/2015/328791. Epub 2015 Feb 10. Gastroenterol Res Pract. 2015. PMID: 25755668 Free PMC article. Review.
-
Potential for therapeutic manipulation of the UPR in disease.Semin Immunopathol. 2013 May;35(3):351-73. doi: 10.1007/s00281-013-0370-z. Epub 2013 Apr 10. Semin Immunopathol. 2013. PMID: 23572207 Free PMC article. Review.
-
Rhubarb extract rebuilding the mucus homeostasis and regulating mucin-associated flora to relieve constipation.Exp Biol Med (Maywood). 2023 Dec;248(23):2449-2463. doi: 10.1177/15353702231211859. Epub 2023 Dec 11. Exp Biol Med (Maywood). 2023. PMID: 38073524 Free PMC article.
-
Evolution and function of the epithelial cell-specific ER stress sensor IRE1β.Mucosal Immunol. 2021 Nov;14(6):1235-1246. doi: 10.1038/s41385-021-00412-8. Epub 2021 Jun 1. Mucosal Immunol. 2021. PMID: 34075183 Free PMC article. Review.
-
Expression of inositol-requiring enzyme 1β is downregulated in azoxymethane/dextran sulfate sodium-induced mouse colonic tumors.Exp Ther Med. 2019 Apr;17(4):3181-3188. doi: 10.3892/etm.2019.7317. Epub 2019 Feb 26. Exp Ther Med. 2019. PMID: 30936991 Free PMC article.
References
-
- Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr. Opin. Pulm. Med. 2006;12:1–6. - PubMed
-
- Ribeiro CMP, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2 + i signaling. The mechanism for the larger agonist-mediated Ca2 + i signals in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005;280:10202–10209. - PubMed
-
- Ribeiro CMP. Chronic airway infection/Inflammation Induces a Ca2+ i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 2005;280:17798–17806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

