A DNA repair BRCA1 estrogen receptor and _targeted therapy in breast cancer
- PMID: 23203101
- PMCID: PMC3509617
- DOI: 10.3390/ijms131114898
A DNA repair BRCA1 estrogen receptor and _targeted therapy in breast cancer
Abstract
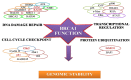
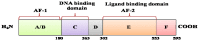
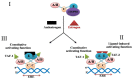
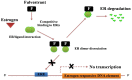
BRCA1 is a key mediator of DNA repair pathways and participates in the maintenance of the genomic integrity of cells. The control of DNA damage repair mechanisms by BRCA1 is of great interest since molecular defects in this pathway may reflect a predictive value in terms of a cell's sensitivity to DNA damaging agents or anticancer drugs. BRCA1 has been found to exhibit a hormone-dependent pattern of expression in breast cells. Wild-type BRCA1 is required for the inhibition of the growth of breast tumor cells in response to the pure steroidal ERα antagonist fulvestrant. Also a loss of BRCA1-mediated transcriptional activation of ERα expression results in increased resistance to ERα antagonists. Platinum-based drugs, poly(ADP-ribose) polymerase (PARP) inhibitors, and their combination are currently included in chemotherapy regimens for breast cancer. Preclinical and clinical studies in a BRCA1-defective setting have recently indicated a rationale for the use of these compounds against hereditary breast cancers. Initial findings indicate that neoadjuvant use of cisplatin results in high rates of complete pathological response in patients with breast cancer who have BRCA1 mutations. Cisplatin produces a better response in triple-negative breast cancer (TNBC) than in non-TNBC diseases in both the neoadjuvant and adjuvant settings. This implies that TNBC cells may harbor a dysfunctional BRCA1 repair pathway.
Figures
Similar articles
-
Pathological complete response after cisplatin neoadjuvant therapy is associated with the downregulation of DNA repair genes in BRCA1-associated triple-negative breast cancers.Onco_target. 2016 Oct 18;7(42):68662-68673. doi: 10.18632/onco_target.11900. Onco_target. 2016. PMID: 27626685 Free PMC article.
-
BRCA1 promoter hypermethylation, 53BP1 protein expression and PARP-1 activity as biomarkers of DNA repair deficit in breast cancer.BMC Cancer. 2013 Nov 5;13:523. doi: 10.1186/1471-2407-13-523. BMC Cancer. 2013. PMID: 24191908 Free PMC article.
-
The Correlation Between PARP1 and BRCA1 in AR Positive Triple-negative Breast Cancer.Int J Biol Sci. 2016 Nov 25;12(12):1500-1510. doi: 10.7150/ijbs.16176. eCollection 2016. Int J Biol Sci. 2016. PMID: 27994514 Free PMC article.
-
BRCA Mutational Status is a Promising Predictive Biomarker for Platinum- based Chemotherapy in Triple-Negative Breast Cancer.Curr Drug _targets. 2020;21(10):962-973. doi: 10.2174/1389450121666200203162541. Curr Drug _targets. 2020. PMID: 32013831 Review.
-
Triple-negative breast cancer and poly(ADP-ribose) polymerase inhibitors.Anticancer Agents Med Chem. 2012 Jul;12(6):672-7. doi: 10.2174/187152012800617759. Anticancer Agents Med Chem. 2012. PMID: 22263793 Review.
Cited by
-
FEN1 is a prognostic biomarker for ER+ breast cancer and associated with tamoxifen resistance through the ERα/cyclin D1/Rb axis.Ann Transl Med. 2021 Feb;9(3):258. doi: 10.21037/atm-20-3068. Ann Transl Med. 2021. PMID: 33708885 Free PMC article.
-
Silencing of CXCR4 sensitizes triple-negative breast cancer cells to cisplatin.Onco_target. 2015 Jan 20;6(2):1020-30. doi: 10.18632/onco_target.2741. Onco_target. 2015. PMID: 25544759 Free PMC article.
-
Platinum-based adjuvant therapy was efficient for triple-negative breast cancer: a meta-analysis from randomized controlled trials.Bioengineered. 2022 Jun;13(6):14827-14839. doi: 10.1080/21655979.2022.2115616. Bioengineered. 2022. PMID: 36278891 Free PMC article.
-
Modulation of BRCA1 mediated DNA damage repair by deregulated ER-α signaling in breast cancers.Am J Cancer Res. 2022 Jan 15;12(1):17-47. eCollection 2022. Am J Cancer Res. 2022. PMID: 35141003 Free PMC article.
-
Predicting chemoinsensitivity in breast cancer with 'omics/digital pathology data fusion.R Soc Open Sci. 2016 Feb 10;3(2):140501. doi: 10.1098/rsos.140501. eCollection 2016 Feb. R Soc Open Sci. 2016. PMID: 26998311 Free PMC article.
References
-
- Rosen E.M., Fan S., Pestell R.G., Goldberg I.D. BRCA1 gene in breast cancer. J. Cell. Physiol. 2003;196:19–41. - PubMed
-
- Hashizume R., Fukuda M., Maeda I., Nishikawa H., Oyake D., Yabuki Y., Ogata H., Ohta T. The RING heterodomer BRCA1-BARD1 is ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 2001;276:14537–14540. - PubMed
-
- Xia Y., Pao G.M., Chen H.W., Verma I.M., Hunter T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 2003;278:5255–5263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

