Inhibition of Hypoxia Inducible Factor Alpha and Astrocyte-Elevated Gene-1 Mediates Cryptotanshinone Exerted Antitumor Activity in Hypoxic PC-3 Cells
- PMID: 23243443
- PMCID: PMC3519236
- DOI: 10.1155/2012/390957
Inhibition of Hypoxia Inducible Factor Alpha and Astrocyte-Elevated Gene-1 Mediates Cryptotanshinone Exerted Antitumor Activity in Hypoxic PC-3 Cells
Erratum in
- Evid Based Complement Alternat Med. 2013;2013:267352
Abstract
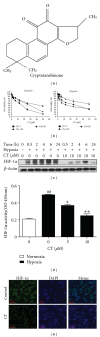
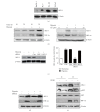
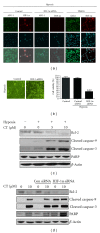
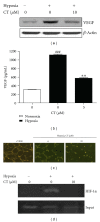
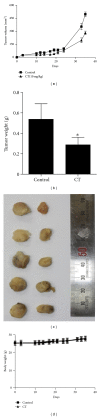
Although cryptotanshinone (CT) was known to exert antitumor activity in several cancers, its molecular mechanism under hypoxia still remains unclear. Here, the roles of AEG-1 and HIF-1α in CT-induced antitumor activity were investigated in hypoxic PC-3 cells. CT exerted cytotoxicity against prostate cancer cells and suppressed HIF-1α accumulation and AEG-1 expression in hypoxic PC-3 cells. Also, AEG-1 was overexpressed in prostate cancer cells. Interestingly, HIF-1α siRNA transfection enhanced the cleavages of caspase-9,3, and PAPR and decreased expression of Bcl-2 and AEG1 induced by CT in hypoxic PC-3 cells. Of note, DMOG enhanced the stability of AEG-1 and HIF-1α during hypoxia. Additionally, CT significantly reduced cellular level of VEGF in PC-3 cells and disturbed tube formation of HUVECs. Consistently, ChIP assay revealed that CT inhibited the binding of HIF-1α to VEGF promoter. Furthermore, CT at 10 mg/kg suppressed the growth of PC-3 cells in BALB/c athymic nude mice by 46.4% compared to untreated control. Consistently, immunohistochemistry revealed decreased expression of Ki-67, CD34, VEGF, carbonic anhydrase IX, and AEG-1 indices in CT-treated group compared to untreated control. Overall, our findings suggest that CT exerts antitumor activity via inhibition of HIF-1α, AEG1, and VEGF as a potent chemotherapeutic agent.
Figures

Similar articles
-
Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells.Biol Pharm Bull. 2011;34(6):850-5. doi: 10.1248/bpb.34.850. Biol Pharm Bull. 2011. PMID: 21628883
-
SPHK/HIF-1α Signaling Pathway Has a Critical Role in Chrysin-Induced Anticancer Activity in Hypoxia-Induced PC-3 Cells.Cells. 2022 Sep 7;11(18):2787. doi: 10.3390/cells11182787. Cells. 2022. PMID: 36139362 Free PMC article.
-
Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic Properties of Melatonin in Hypoxic PC-3 Prostate Cancer Cells.J Cancer. 2015 Jan 1;6(1):19-28. doi: 10.7150/jca.9591. eCollection 2015. J Cancer. 2015. PMID: 25553085 Free PMC article.
-
Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells.Biochem Pharmacol. 2013 May 1;85(9):1278-87. doi: 10.1016/j.bcp.2013.02.009. Epub 2013 Feb 14. Biochem Pharmacol. 2013. PMID: 23416116
-
Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line.J Biol Chem. 2002 Dec 20;277(51):50081-6. doi: 10.1074/jbc.M201095200. Epub 2002 Oct 24. J Biol Chem. 2002. PMID: 12401798
Cited by
-
Molecular Mechanism of Tanshinone against Prostate Cancer.Molecules. 2022 Aug 30;27(17):5594. doi: 10.3390/molecules27175594. Molecules. 2022. PMID: 36080361 Free PMC article. Review.
-
Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review.Cells. 2021 Jun 15;10(6):1497. doi: 10.3390/cells10061497. Cells. 2021. PMID: 34203598 Free PMC article. Review.
-
Astrocyte elevated gene-1: a novel independent prognostic biomarker for metastatic ovarian tumors.Tumour Biol. 2014 Apr;35(4):3079-85. doi: 10.1007/s13277-013-1400-0. Tumour Biol. 2014. PMID: 24234336
-
AEG-1/MTDH/LYRIC, the beginning: initial cloning, structure, expression profile, and regulation of expression.Adv Cancer Res. 2013;120:1-38. doi: 10.1016/B978-0-12-401676-7.00001-2. Adv Cancer Res. 2013. PMID: 23889986 Free PMC article. Review.
-
Progress of cancer research on astrocyte elevated gene-1/Metadherin (Review).Oncol Lett. 2014 Aug;8(2):493-501. doi: 10.3892/ol.2014.2231. Epub 2014 Jun 5. Oncol Lett. 2014. PMID: 25009642 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer Journal for Clinicians. 2009;59(4):225–249. - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. European Journal of Cancer. 2010;46(4):765–781. - PubMed
-
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. Ca-A Cancer Journal for Clinicians. 2002;52(1):23–47. - PubMed
-
- Kish JA, Bukkapatnam R, Palazzo F. The treatment challenge of hormone-refractory prostate cancer. Cancer Control. 2001;8(6):487–495. - PubMed
-
- Brawley OW, Barnes S, Parnes H. The future of prostate cancer prevention. Annals of the New York Academy of Sciences. 2001;952:145–152. - PubMed
LinkOut - more resources
Full Text Sources

