Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain
- PMID: 23272051
- PMCID: PMC3522681
- DOI: 10.1371/journal.pone.0049789
Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain
Abstract
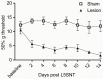
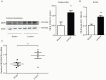
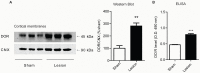
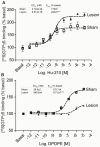
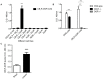
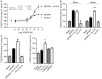
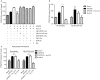
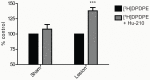
The diversity of receptor signaling is increased by receptor heteromerization leading to dynamic regulation of receptor function. While a number of studies have demonstrated that family A G-protein-coupled receptors are capable of forming heteromers in vitro, the role of these heteromers in normal physiology and disease has been poorly explored. In this study, direct interactions between CB(1) cannabinoid and delta opioid receptors in the brain were examined. Additionally, regulation of heteromer levels and signaling in a rodent model of neuropathic pain was explored. First we examined changes in the expression, function and interaction of these receptors in the cerebral cortex of rats with a peripheral nerve lesion that resulted in neuropathic pain. We found that, following the peripheral nerve lesion, the expression of both cannabinoid type 1 receptor (CB(1)R) and the delta opioid receptor (DOR) are increased in select brain regions. Concomitantly, an increase in CB(1)R activity and decrease in DOR activity was observed. We hypothesize that this decrease in DOR activity could be due to heteromeric interactions between these two receptors. Using a CB(1)R-DOR heteromer-specific antibody, we found increased levels of CB(1)R-DOR heteromer protein in the cortex of neuropathic animals. We subsequently examined the functionality of these heteromers by testing whether low, non-signaling doses of CB(1)R ligands influenced DOR signaling in the cortex. We found that, in cortical membranes from animals that experienced neuropathic pain, non-signaling doses of CB(1)R ligands significantly enhanced DOR activity. Moreover, this activity is selectively blocked by a heteromer-specific antibody. Together, these results demonstrate an important role for CB(1)R-DOR heteromers in altered cortical function of DOR during neuropathic pain. Moreover, they suggest the possibility that a novel heteromer-directed therapeutic strategy for enhancing DOR activity, could potentially be employed to reduce anxiety associated with chronic pain.
Conflict of interest statement
Figures

Similar articles
-
Revolution in GPCR signalling: opioid receptor heteromers as novel therapeutic _targets: IUPHAR review 10.Br J Pharmacol. 2014 Sep;171(18):4155-76. doi: 10.1111/bph.12798. Br J Pharmacol. 2014. PMID: 24916280 Free PMC article. Review.
-
Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons.PLoS One. 2012;7(1):e29239. doi: 10.1371/journal.pone.0029239. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235275 Free PMC article.
-
Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons.Mol Pharmacol. 2012 Feb;81(2):264-72. doi: 10.1124/mol.111.072702. Epub 2011 Nov 9. Mol Pharmacol. 2012. PMID: 22072818 Free PMC article.
-
Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking.J Pharmacol Exp Ther. 2011 Jun;337(3):868-75. doi: 10.1124/jpet.111.179093. Epub 2011 Mar 21. J Pharmacol Exp Ther. 2011. PMID: 21422164 Free PMC article.
-
Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities.Br J Pharmacol. 2015 Jan;172(2):375-87. doi: 10.1111/bph.12663. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24571499 Free PMC article. Review.
Cited by
-
Polypharmacological Approaches for CNS Diseases: Focus on Endocannabinoid Degradation Inhibition.Cells. 2022 Jan 29;11(3):471. doi: 10.3390/cells11030471. Cells. 2022. PMID: 35159280 Free PMC article. Review.
-
Molecular Pharmacology of δ-Opioid Receptors.Pharmacol Rev. 2016 Jul;68(3):631-700. doi: 10.1124/pr.114.008979. Pharmacol Rev. 2016. PMID: 27343248 Free PMC article. Review.
-
Revolution in GPCR signalling: opioid receptor heteromers as novel therapeutic _targets: IUPHAR review 10.Br J Pharmacol. 2014 Sep;171(18):4155-76. doi: 10.1111/bph.12798. Br J Pharmacol. 2014. PMID: 24916280 Free PMC article. Review.
-
Intra-accumbal Cannabinoid Agonist Attenuated Reinstatement but not Extinction Period of Morphine-Induced Conditioned Place Preference; Evidence for Different Characteristics of Extinction Period and Reinstatement.Neurochem Res. 2017 Nov;42(11):3321-3330. doi: 10.1007/s11064-017-2374-x. Epub 2017 Aug 5. Neurochem Res. 2017. PMID: 28780734
-
The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization.Mol Pharmacol. 2013 Jul;84(1):158-69. doi: 10.1124/mol.113.084780. Epub 2013 Apr 30. Mol Pharmacol. 2013. PMID: 23632086 Free PMC article. Review.
References
-
- Finnerup NB, Sindrup SH, Jensen TS (2010) The evidence for pharmacological treatment of neuropathic pain. Pain 150: 573–581. - PubMed
-
- O'Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122: S22–32. - PubMed
-
- Bausch SB, Patterson TA, Appleyard SM, Chavkin C (1995) Immunocytochemical localization of delta opioid receptors in mouse brain. J Chem Neuroanat 8: 175–189. - PubMed
-
- Hohmann AG, Briley EM, Herkenham M (1999) Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res 822: 17–25. - PubMed
-
- Maldonado R, Valverde O (2003) Participation of the opioid system in cannabinoid-induced antinociception and emotional-like responses. Eur Neuropsychopharmacol 13: 401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

