Virus-induced ER stress and the unfolded protein response
- PMID: 23293645
- PMCID: PMC3531707
- DOI: 10.3389/fpls.2012.00293
Virus-induced ER stress and the unfolded protein response
Abstract
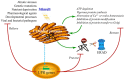
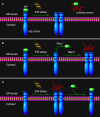
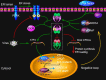
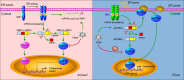
The accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum (ER) results in ER stress that triggers cytoprotective signaling pathways, termed the unfolded protein response (UPR), to restore and maintain homeostasis in the ER or to induce apoptosis if ER stress remains unmitigated. The UPR signaling network encompasses three core elements, i.e., PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring protein-1 (IRE1). Activation of these three branch pathways of the UPR leads to the translation arrest and degradation of misfolded proteins, the expression of ER molecular chaperones, and the expansion of the ER membrane to decrease the load of proteins and increase the protein-folding capacity in the ER. Recently, the essential roles of the UPR have been implicated in a number of mammalian diseases, particularly viral diseases. In virus-infected cells, the cellular translation machinery is hijacked by the infecting virus to produce large amounts of viral proteins, which inevitably perturbs ER homeostasis and causes ER stress. This review summarizes current knowledge about the UPR signaling pathways, highlights two identified UPR pathways in plants, and discuss progress in elucidating the UPR in virus-infected cells and its functional roles in viral infection.
Keywords: ER stress; endoplasmic reticulum; signaling transduction; unfolded protein response; virus.
Figures

Similar articles
-
The Human Cytomegalovirus Endoplasmic Reticulum-Resident Glycoprotein UL148 Activates the Unfolded Protein Response.J Virol. 2018 Sep 26;92(20):e00896-18. doi: 10.1128/JVI.00896-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045994 Free PMC article.
-
Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - _targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Review.
-
The PERK Arm of the Unfolded Protein Response Negatively Regulates Transmissible Gastroenteritis Virus Replication by Suppressing Protein Translation and Promoting Type I Interferon Production.J Virol. 2018 Jul 17;92(15):e00431-18. doi: 10.1128/JVI.00431-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769338 Free PMC article.
-
A small molecule UPR modulator for diabetes identified by high throughput screening.Acta Pharm Sin B. 2021 Dec;11(12):3983-3993. doi: 10.1016/j.apsb.2021.05.018. Epub 2021 Jun 16. Acta Pharm Sin B. 2021. PMID: 35024320 Free PMC article.
-
Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review.Int J Mol Sci. 2023 Sep 14;24(18):14066. doi: 10.3390/ijms241814066. Int J Mol Sci. 2023. PMID: 37762367 Free PMC article. Review.
Cited by
-
Novel Therapeutic Nutrients Molecules That Protect against Zika Virus Infection with a Special Note on Palmitoleate.Nutrients. 2022 Dec 27;15(1):124. doi: 10.3390/nu15010124. Nutrients. 2022. PMID: 36615782 Free PMC article. Review.
-
Classical Swine Fever Virus Infection Induces Endoplasmic Reticulum Stress-Mediated Autophagy to Sustain Viral Replication in vivo and in vitro.Front Microbiol. 2019 Nov 12;10:2545. doi: 10.3389/fmicb.2019.02545. eCollection 2019. Front Microbiol. 2019. PMID: 31798542 Free PMC article.
-
Sugarcane streak mosaic virus P1 protein inhibits unfolded protein response through direct suppression of bZIP60U splicing.PLoS Pathog. 2023 Oct 26;19(10):e1011738. doi: 10.1371/journal.ppat.1011738. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37883577 Free PMC article.
-
Crosstalk between cGAS-STING pathway and autophagy in cancer immunity.Front Immunol. 2023 Mar 1;14:1139595. doi: 10.3389/fimmu.2023.1139595. eCollection 2023. Front Immunol. 2023. PMID: 36936940 Free PMC article. Review.
-
Comparative proteomic analysis of a membrane-enriched fraction from flag leaves reveals responses to chemical hybridization agent SQ-1 in wheat.Front Plant Sci. 2015 Aug 26;6:669. doi: 10.3389/fpls.2015.00669. eCollection 2015. Front Plant Sci. 2015. PMID: 26379693 Free PMC article.
References
-
- Baltzis D., Qu L. K., Papadopoulou S., Blais J. D., Bell J. C., Sonenberg N., et al. (2004). Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 78, 12747–12761 10.1128/JVI.78.23.12747-12761.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

