Hyperoxia causes regression of vitreous neovascularization by downregulating VEGF/VEGFR2 pathway
- PMID: 23307955
- PMCID: PMC3564450
- DOI: 10.1167/iovs.12-11291
Hyperoxia causes regression of vitreous neovascularization by downregulating VEGF/VEGFR2 pathway
Abstract
Purpose: Neovascularization (NV) is a sight-threatening complication of retinal ischemia in diabetes, retinal vein occlusion, and retinopathy of prematurity. Current treatment modalities, including laser photocoagulation and repeated intraocular injection of VEGF antagonists, are invasive and not always effective, and may carry side effects. We studied the use of hyperoxia as an alternative therapeutic strategy for regressing established vitreous NV in a mouse model of oxygen-induced ischemic retinopathy.
Methods: Hyperoxia treatment (HT, 75% oxygen) was initiated on postnatal day (P)17 after the onset of vitreous NV. Immunohistochemistry and quantitative PCR were used to assess retinal vascular changes in relation to apoptosis, and expression of VEGFR2 and inflammatory molecules. Effects of intravitreal injections of VEGF-A, VEGF-E, PlGF-1, and VEGF trap were also studied.
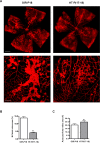
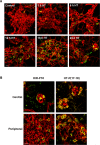
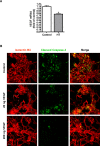
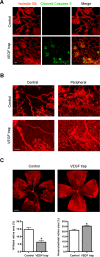
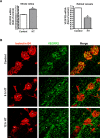
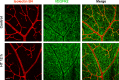
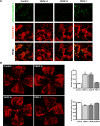
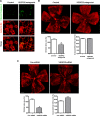
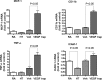
Results: HT selectively reduced NV by 70% within 24 hours. It robustly increased the level of cleaved caspase-3 in the vitreous NV between 6 and 18 hours and promoted infiltration of macrophage/microglial cells. The HT-induced apoptosis was preceded by a significant reduction in VEGFR2 expression within the NV and an increase in VEGFR2 within the surrounding neural tissue. Intravitreal VEGF-A and VEGF-E (VEGFR2 agonist) but not PlGF-1 (VEGFR1 agonist) prevented HT-induced apoptosis and regression of NV. In contrast, VEGF trap and VEGFR2 blockers mimicked the effect of HT. However, intravitreal VEGF trap induced increases in inflammatory molecules while HT did not have such unwanted effect.
Conclusions: HT may be clinically useful to specifically treat proliferative NV in ischemic retinopathy.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model.Invest Ophthalmol Vis Sci. 2011 Aug 11;52(9):6384-95. doi: 10.1167/iovs.11-7666. Invest Ophthalmol Vis Sci. 2011. PMID: 21705685 Free PMC article.
-
Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog.Invest Ophthalmol Vis Sci. 2011 Jun 8;52(7):4039-47. doi: 10.1167/iovs.10-6798. Invest Ophthalmol Vis Sci. 2011. PMID: 21357392 Free PMC article.
-
Inhibition of experimental choroidal neovascularization in mice by anti-VEGFA/VEGFR2 or non-specific siRNA.Exp Eye Res. 2010 Sep;91(3):433-9. doi: 10.1016/j.exer.2010.06.019. Epub 2010 Jul 1. Exp Eye Res. 2010. PMID: 20599960
-
Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis).Trans Am Ophthalmol Soc. 2010 Dec;108:96-119. Trans Am Ophthalmol Soc. 2010. PMID: 21212851 Free PMC article. Review.
-
Sensitivity of different vascular beds in the eye to neovascularization and blood-retinal barrier breakdown in VEGF transgenic mice.Adv Exp Med Biol. 2000;476:129-38. doi: 10.1007/978-1-4615-4221-6_11. Adv Exp Med Biol. 2000. PMID: 10949661 Review.
Cited by
-
Intravitreal administration of recombinant human opticin protects against hyperoxia-induced pre-retinal neovascularization.Exp Eye Res. 2022 Feb;215:108908. doi: 10.1016/j.exer.2021.108908. Epub 2021 Dec 23. Exp Eye Res. 2022. PMID: 34954204 Free PMC article.
-
Alterations of retinal vasculature in cystathionine-β-synthase heterozygous mice: a model of mild to moderate hyperhomocysteinemia.Am J Pathol. 2014 Sep;184(9):2573-85. doi: 10.1016/j.ajpath.2014.05.018. Epub 2014 Jul 10. Am J Pathol. 2014. PMID: 25016930 Free PMC article.
-
Diabetic Macular Edema: From Old Concepts to New Therapeutic Avenues.Med Hypothesis Discov Innov Ophthalmol. 2015 Winter;4(4):130-135. Med Hypothesis Discov Innov Ophthalmol. 2015. PMID: 27800500 Free PMC article. Review.
-
TWEAK/Fn14 pathway is a novel mediator of retinal neovascularization.Invest Ophthalmol Vis Sci. 2014 Feb 10;55(2):801-13. doi: 10.1167/iovs.13-12812. Invest Ophthalmol Vis Sci. 2014. PMID: 24408972 Free PMC article.
-
Therapeutic Effect of Traditional Chinese Medicine on a Rat Model of Branch Retinal Vein Occlusion.J Ophthalmol. 2019 Feb 18;2019:9521379. doi: 10.1155/2019/9521379. eCollection 2019. J Ophthalmol. 2019. PMID: 30906588 Free PMC article.
References
-
- Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007; 27: 816–824 - PubMed
-
- Tolentino MJ. Current molecular understanding and future treatment strategies for pathologic ocular neovascularization. Curr Mol Med. 2009; 9: 973–981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

