Hydrogel drug delivery system with predictable and tunable drug release and degradation rates
- PMID: 23345437
- PMCID: PMC3568318
- DOI: 10.1073/pnas.1215498110
Hydrogel drug delivery system with predictable and tunable drug release and degradation rates
Abstract
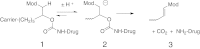
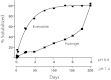
Many drugs and drug candidates are suboptimal because of short duration of action. For example, peptides and proteins often have serum half-lives of only minutes to hours. One solution to this problem involves conjugation to circulating carriers, such as PEG, that retard kidney filtration and hence increase plasma half-life of the attached drug. We recently reported an approach to half-life extension that uses sets of self-cleaving linkers to attach drugs to macromolecular carriers. The linkers undergo β-eliminative cleavage to release the native drug with predictable half-lives ranging from a few hours to over 1 y; however, half-life extension becomes limited by the renal elimination rate of the circulating carrier. An approach to overcoming this constraint is to use noncirculating, biodegradable s.c. implants as drug carriers that are stable throughout the duration of drug release. Here, we use β-eliminative linkers to both tether drugs to and cross-link PEG hydrogels, and demonstrate tunable drug release and hydrogel erosion rates over a very wide range. By using one β-eliminative linker to tether a drug to the hydrogel, and another β-eliminative linker with a longer half-life to control polymer degradation, the system can be coordinated to release the drug before the gel undergoes complete erosion. The practical utility is illustrated by a PEG hydrogel-exenatide conjugate that should allow once-a-month administration, and results indicate that the technology may serve as a generic platform for tunable ultralong half-life extension of potent therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6211-6. doi: 10.1073/pnas.1117147109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474378 Free PMC article.
-
Hydrogel Drug Delivery System Using Self-Cleaving Covalent Linkers for Once-a-Week Administration of Exenatide.Bioconjug Chem. 2016 May 18;27(5):1210-5. doi: 10.1021/acs.bioconjchem.5b00690. Epub 2016 Mar 22. Bioconjug Chem. 2016. PMID: 26930186
-
Biodegradable tetra-PEG hydrogels as carriers for a releasable drug delivery system.Bioconjug Chem. 2015 Feb 18;26(2):270-8. doi: 10.1021/bc5005476. Epub 2015 Jan 13. Bioconjug Chem. 2015. PMID: 25584814
-
Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications.Eur J Pharm Biopharm. 2014 Nov;88(3):575-85. doi: 10.1016/j.ejpb.2014.07.005. Epub 2014 Aug 1. Eur J Pharm Biopharm. 2014. PMID: 25092423 Review.
-
Advances in PEGylation of important biotech molecules: delivery aspects.Expert Opin Drug Deliv. 2008 Apr;5(4):371-83. doi: 10.1517/17425247.5.4.371. Expert Opin Drug Deliv. 2008. PMID: 18426380 Review.
Cited by
-
Cobalt ions-derived nanoenzyme array for endosseous neural network reconstruction and osseointegration.Bioact Mater. 2024 Aug 14;42:1-17. doi: 10.1016/j.bioactmat.2024.08.005. eCollection 2024 Dec. Bioact Mater. 2024. PMID: 39246698 Free PMC article.
-
The Synthesis of RGD-functionalized Hydrogels as a Tool for Therapeutic Applications.J Vis Exp. 2016 Oct 7;(116):54445. doi: 10.3791/54445. J Vis Exp. 2016. PMID: 27768038 Free PMC article.
-
4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery.Pharmaceuticals (Basel). 2022 Oct 19;15(10):1282. doi: 10.3390/ph15101282. Pharmaceuticals (Basel). 2022. PMID: 36297394 Free PMC article. Review.
-
Application of microfluidic chip technology in pharmaceutical analysis: A review.J Pharm Anal. 2019 Aug;9(4):238-247. doi: 10.1016/j.jpha.2018.12.001. Epub 2018 Dec 6. J Pharm Anal. 2019. PMID: 31452961 Free PMC article.
-
Tissue-reactive drugs enable materials-free local depots.J Control Release. 2022 Mar;343:142-151. doi: 10.1016/j.jconrel.2022.01.023. Epub 2022 Jan 22. J Control Release. 2022. PMID: 35077743 Free PMC article.
References
-
- Greenwald RB, et al. Controlled release of proteins from their poly(ethylene glycol) conjugates: Drug delivery systems employing 1,6-elimination. Bioconjug Chem. 2003;14(2):395–403. - PubMed
-
- Filpula D, Zhao H. Releasable PEGylation of proteins with customized linkers. Adv Drug Deliv Rev. 2008;60(1):29–49. - PubMed
-
- Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym Chem. 2011;2(7):1442–1448.
-
- Fishburn CS. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97(10):4167–4183. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

