Cholic acid for hepatic steatosis in patients with lipodystrophy: a randomized, controlled trial
- PMID: 23447519
- PMCID: PMC3902034
- DOI: 10.1530/EJE-12-0969
Cholic acid for hepatic steatosis in patients with lipodystrophy: a randomized, controlled trial
Abstract
Objective: Hepatic steatosis is a common complication in patients with lipodystrophies and can lead to cirrhosis. There is no proven effective therapy for hepatic steatosis, but cholic acid (CA), a farnesoid X receptor agonist, has previously been shown to reduce hepatic triglyceride (TG) content in mice and serum TG in humans. Our objective was to assess clinical efficacy and tolerability of CA therapy in patients with lipodystrophy and hepatic steatosis.
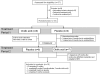
Design: A randomized, double-blind, placebo-controlled, crossover study.
Methods: Eighteen patients with genetic or autoimmune lipodystrophies and elevated hepatic TG content participated in the study. The intervention was CA (15 mg/kg per day) compared with placebo for a period of 6 months each. Hepatic TG content, the primary outcome variable, was measured with (1)H magnetic resonance spectroscopy at baseline and at 3 and 6 months during each study period. Levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), and TG were secondary end points of the study.
Results: Compared with placebo, CA did not reduce (median (interquartile range) hepatic TG content (14.8% (9.4-19.0%) vs 15.9% (10.5-26.5%) respectively; P=0.42) or serum TG ((340 mg/dl (233-433 mg/dl) vs 390 mg/dl (233-595 mg/dl) respectively; P=0.45)). CA therapy also did not change AST, ALT, or GGT levels. Two patients developed diarrhea and excessive flatus while taking CA and these symptoms resolved after reducing the dose of CA.
Conclusion: CA was well tolerated but did not reduce hepatic TG content in patients with lipodystrophy.
Conflict of interest statement
Figures


Similar articles
-
Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: a double-blind, randomized, placebo-controlled trial.Eur J Nutr. 2017 Aug;56(5):1993-2002. doi: 10.1007/s00394-016-1318-6. Epub 2016 Oct 14. Eur J Nutr. 2017. PMID: 27743130 Clinical Trial.
-
Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study.Hepatology. 1996 Jun;23(6):1464-7. doi: 10.1002/hep.510230624. Hepatology. 1996. PMID: 8675165
-
The liver diseases of lipodystrophy: the long-term effect of leptin treatment.J Hepatol. 2013 Jul;59(1):131-7. doi: 10.1016/j.jhep.2013.02.007. Epub 2013 Feb 21. J Hepatol. 2013. PMID: 23439261 Free PMC article. Clinical Trial.
-
A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase.Clin Chem Lab Med. 2013 Oct;51(10):1997-2007. doi: 10.1515/cclm-2013-0096. Clin Chem Lab Med. 2013. PMID: 24072574 Review.
-
Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.Cochrane Database Syst Rev. 2013 Dec 27;(12):CD008623. doi: 10.1002/14651858.CD008623.pub2. Cochrane Database Syst Rev. 2013. PMID: 24374462 Review.
Cited by
-
Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging.Am J Cardiol. 2013 Oct 1;112(7):1019-24. doi: 10.1016/j.amjcard.2013.05.036. Epub 2013 Jun 22. Am J Cardiol. 2013. PMID: 23800548 Free PMC article.
-
Treatment Options for Lipodystrophy in Children.Front Endocrinol (Lausanne). 2022 May 4;13:879979. doi: 10.3389/fendo.2022.879979. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600578 Free PMC article. Review.
-
Update on Therapeutic Options in Lipodystrophy.Curr Diab Rep. 2018 Oct 29;18(12):139. doi: 10.1007/s11892-018-1100-7. Curr Diab Rep. 2018. PMID: 30370487 Review.
-
Dunnigan lipodystrophy syndrome: French National Diagnosis and Care Protocol (PNDS; Protocole National de Diagnostic et de Soins).Orphanet J Rare Dis. 2022 Apr 19;17(Suppl 1):170. doi: 10.1186/s13023-022-02308-7. Orphanet J Rare Dis. 2022. PMID: 35440056 Free PMC article.
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic _targets.Signal Transduct _target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct _target Ther. 2024. PMID: 38664391 Free PMC article. Review.
References
-
- Garg A. Acquired and genetic lipodystrophies. N Engl J Med. 2004;350:1220–1234. - PubMed
-
- Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine. 2003;82:129–146. - PubMed
-
- Cauble MS, Gilroy R, Sorrell MF, Mailliard ME, Sudan DL, Anderson JC, Wisecarver JL, Balakrishnan S, Larsen JL. Lipoatrophic diabetes and end-stage liver disease secondary to nonalcoholic steatohepatitis with recurrence after liver transplantation. Transplantation. 2001;71:892–895. - PubMed
-
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. - PubMed
-
- Simha V, Szczepaniak LS, Wagner AJ, DePaoli AM, Garg A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care. 2003;26:30–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

