Telomeres and age-related disease: how telomere biology informs clinical paradigms
- PMID: 23454763
- PMCID: PMC3673231
- DOI: 10.1172/JCI66370
Telomeres and age-related disease: how telomere biology informs clinical paradigms
Abstract
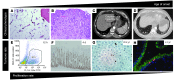
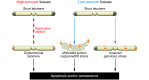
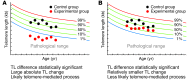
Telomere length shortens with age and predicts the onset of replicative senescence. Recently, short telomeres have been linked to the etiology of degenerative diseases such as idiopathic pulmonary fibrosis, bone marrow failure, and cryptogenic liver cirrhosis. These disorders have recognizable clinical manifestations, and the telomere defect explains their genetics and informs the approach to their treatment. Here, I review how telomere biology has become intimately connected to clinical paradigms both for understanding pathophysiology and for individualizing therapy decisions. I also critically examine nuances of interpreting telomere length measurement in clinical studies.
Figures
Similar articles
-
The short and long telomere syndromes: paired paradigms for molecular medicine.Curr Opin Genet Dev. 2015 Aug;33:1-9. doi: 10.1016/j.gde.2015.06.004. Epub 2015 Jul 29. Curr Opin Genet Dev. 2015. PMID: 26232116 Free PMC article. Review.
-
Heart-Breaking Telomeres.Circ Res. 2018 Sep 14;123(7):787-802. doi: 10.1161/CIRCRESAHA.118.312202. Circ Res. 2018. PMID: 30355079 Review.
-
Aging, Cancer, and Inflammation: The Telomerase Connection.Int J Mol Sci. 2024 Aug 5;25(15):8542. doi: 10.3390/ijms25158542. Int J Mol Sci. 2024. PMID: 39126110 Free PMC article. Review.
-
Telomere length variations in aging and age-related diseases.Curr Aging Sci. 2014;7(3):161-7. doi: 10.2174/1874609808666150122153151. Curr Aging Sci. 2014. PMID: 25612739 Review.
-
Investigation of telomere related gene mutations in idiopathic pulmonary fibrosis.Mol Biol Rep. 2020 Oct;47(10):7851-7860. doi: 10.1007/s11033-020-05861-1. Epub 2020 Oct 1. Mol Biol Rep. 2020. PMID: 33006015 Clinical Trial.
Cited by
-
Simultaneous Liver and Kidney Transplantation in a Patient With Telomere Biology Disorder: A Case Study.J Clin Exp Hepatol. 2024 May-Jun;14(3):101355. doi: 10.1016/j.jceh.2024.101355. Epub 2024 Feb 1. J Clin Exp Hepatol. 2024. PMID: 38389866
-
Association between Rheumatic Disease Comorbidity Index and factors of poor prognosis in a cohort of 280 patients with rheumatoid arthritis.BMC Rheumatol. 2022 Dec 21;6(1):78. doi: 10.1186/s41927-022-00308-5. BMC Rheumatol. 2022. PMID: 36539858 Free PMC article.
-
Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus).Animals (Basel). 2021 Nov 16;11(11):3276. doi: 10.3390/ani11113276. Animals (Basel). 2021. PMID: 34828008 Free PMC article.
-
Chromosome-specific telomere lengths and the minimal functional telomere revealed by nanopore sequencing.Genome Res. 2022 Apr;32(4):616-628. doi: 10.1101/gr.275868.121. Epub 2021 Oct 26. Genome Res. 2022. PMID: 34702734 Free PMC article.
-
Mechanistic links between aging and lung fibrosis.Biogerontology. 2013 Dec;14(6):609-15. doi: 10.1007/s10522-013-9451-6. Epub 2013 Aug 9. Biogerontology. 2013. PMID: 23929205 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

