The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is _targeted by neutralizing antibodies
- PMID: 23468491
- PMCID: PMC3648152
- DOI: 10.1128/JVI.00128-13
The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is _targeted by neutralizing antibodies
Abstract
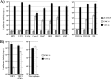
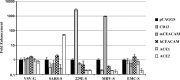
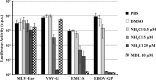
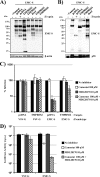
The novel human coronavirus EMC (hCoV-EMC), which recently emerged in Saudi Arabia, is highly pathogenic and could pose a significant threat to public health. The elucidation of hCoV-EMC interactions with host cells is critical to our understanding of the pathogenesis of this virus and to the identification of _targets for antiviral intervention. Here we investigated the viral and cellular determinants governing hCoV-EMC entry into host cells. We found that the spike protein of hCoV-EMC (EMC-S) is incorporated into lentiviral particles and mediates transduction of human cell lines derived from different organs, including the lungs, kidneys, and colon, as well as primary human macrophages. Expression of the known coronavirus receptors ACE2, CD13, and CEACAM1 did not facilitate EMC-S-driven transduction, suggesting that hCoV-EMC uses a novel receptor for entry. Directed protease expression and inhibition analyses revealed that TMPRSS2 and endosomal cathepsins activate EMC-S for virus-cell fusion and constitute potential _targets for antiviral intervention. Finally, EMC-S-driven transduction was abrogated by serum from an hCoV-EMC-infected patient, indicating that EMC-S-specific neutralizing antibodies can be generated in patients. Collectively, our results indicate that hCoV-EMC uses a novel receptor for protease-activated entry into human cells and might be capable of extrapulmonary spread. In addition, they define TMPRSS2 and cathepsins B and L as potential _targets for intervention and suggest that neutralizing antibodies contribute to the control of hCoV-EMC infection.
Figures

Similar articles
-
TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral _target cells in the respiratory epithelium.J Virol. 2013 Jun;87(11):6150-60. doi: 10.1128/JVI.03372-12. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536651 Free PMC article.
-
Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response.J Virol. 2011 May;85(9):4122-34. doi: 10.1128/JVI.02232-10. Epub 2011 Feb 16. J Virol. 2011. PMID: 21325420 Free PMC article.
-
Severe fever with thrombocytopenia virus glycoproteins are _targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines.J Virol. 2013 Apr;87(8):4384-94. doi: 10.1128/JVI.02628-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388721 Free PMC article.
-
Mechanisms of coronavirus cell entry mediated by the viral spike protein.Viruses. 2012 Jun;4(6):1011-33. doi: 10.3390/v4061011. Epub 2012 Jun 20. Viruses. 2012. PMID: 22816037 Free PMC article. Review.
-
Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence.Viruses. 2012 Apr;4(4):557-80. doi: 10.3390/v4040557. Epub 2012 Apr 12. Viruses. 2012. PMID: 22590686 Free PMC article. Review.
Cited by
-
Adaptive Immunity to Viruses: What Did We Learn from SARS-CoV-2 Infection?Int J Mol Sci. 2022 Nov 12;23(22):13951. doi: 10.3390/ijms232213951. Int J Mol Sci. 2022. PMID: 36430430 Free PMC article. Review.
-
Localization of Cell Receptor-Related Genes of SARS-CoV-2 in the Kidney through Single-Cell Transcriptome Analysis.Kidney Dis (Basel). 2020 Jul;6(4):258-270. doi: 10.1159/000508162. Epub 2020 May 19. Kidney Dis (Basel). 2020. PMID: 32903321 Free PMC article.
-
Proper Management of People with Obesity during the COVID-19 Pandemic.J Obes Metab Syndr. 2020 Jun 30;29(2):84-98. doi: 10.7570/jomes20056. J Obes Metab Syndr. 2020. PMID: 32544885 Free PMC article.
-
Coronavirus and influenza virus proteolytic priming takes place in tetraspanin-enriched membrane microdomains.J Virol. 2015 Jun;89(11):6093-104. doi: 10.1128/JVI.00543-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833045 Free PMC article.
-
Circular RNAs as emerging regulators in COVID-19 pathogenesis and progression.Front Immunol. 2022 Nov 9;13:980231. doi: 10.3389/fimmu.2022.980231. eCollection 2022. Front Immunol. 2022. PMID: 36439162 Free PMC article. Review.
References
-
- Holmes KV. 2001. Coronaviruses, p 1187–1203 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA
-
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

