Contributions of the Insulin/Insulin-Like Growth Factor-1 Axis to Diabetic Osteopathy
- PMID: 23484069
- PMCID: PMC3593087
- DOI: 10.4172/2155-6156.S1-003
Contributions of the Insulin/Insulin-Like Growth Factor-1 Axis to Diabetic Osteopathy
Abstract
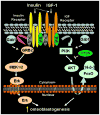
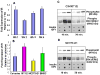
Recent studies in diabetic humans and rodent models of diabetes have identified osteopathy as a serious complication of type 1 (T1D) and type 2 (T2D) diabetes. Accumulating evidence suggests that disruption of insulin and insulin-like growth factor 1 (IGF-1) homeostasis in the diabetic condition may be responsible for the observed skeletal deficits. Indeed, replacement of insulin or IGF-1 in rodent models of T1D results in significant improvement in bone healing despite ongoing moderate to severe hyperglycemia. Insulin and IGF-1 act through distinct receptors. Mice in which the receptor for insulin or IGF-1 is selectively deleted from osteoblast lineages show skeletal deficits. Despite acting through distinct receptors, insulin and IGF-1 exert their cellular activities via conserved intracellular signaling proteins. Genetic manipulation of these signaling proteins, such as insulin receptor substrate (IRS)-1 and -2, Protein Kinase B (Akt), and MAPK/ERK kinase (MEK), has uncovered a significant role for these signal transduction pathways in skeletal homeostasis. In addition to effects on skeletal physiology via canonical signaling pathways, insulin and IGF-1 may crosstalk with wingless-int. (Wnt) and bone morphogenic protein 2 (BMP-2) signaling pathways in cells of the osteoblast lineage and thereby promote skeletal development. In this review, a discussion is presented regarding the role of insulin and IGF-1 in skeletal physiology and disruptions of this axis that occur in the diabetic condition which could underlie many of the skeletal pathologies observed.
Figures

Similar articles
-
Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: differential signalling via Akt and ERK.Cell Biochem Funct. 2012 Jun;30(4):297-302. doi: 10.1002/cbf.2801. Epub 2012 Jan 17. Cell Biochem Funct. 2012. PMID: 22249904
-
IGF-I and insulin receptor signal transduction in trout muscle cells.Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290(6):R1683-90. doi: 10.1152/ajpregu.00294.2005. Epub 2006 Jan 26. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16439672
-
Progesterone receptor-B regulation of insulin-like growth factor-stimulated cell migration in breast cancer cells via insulin receptor substrate-2.Mol Cancer Res. 2008 Sep;6(9):1491-8. doi: 10.1158/1541-7786.MCR-07-2173. Mol Cancer Res. 2008. PMID: 18819936 Free PMC article.
-
TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease.Bone Res. 2016 Apr 26;4:16009. doi: 10.1038/boneres.2016.9. eCollection 2016. Bone Res. 2016. PMID: 27563484 Free PMC article. Review.
-
The IGF-1 Signaling Pathway in Viral Infections.Viruses. 2021 Jul 29;13(8):1488. doi: 10.3390/v13081488. Viruses. 2021. PMID: 34452353 Free PMC article. Review.
Cited by
-
Diabetes pharmacotherapy and effects on the musculoskeletal system.Diabetes Metab Res Rev. 2019 Feb;35(2):e3100. doi: 10.1002/dmrr.3100. Epub 2018 Dec 20. Diabetes Metab Res Rev. 2019. PMID: 30467957 Free PMC article. Review.
-
Activation of mTOR: a culprit of Alzheimer's disease?Neuropsychiatr Dis Treat. 2015 Apr 9;11:1015-30. doi: 10.2147/NDT.S75717. eCollection 2015. Neuropsychiatr Dis Treat. 2015. PMID: 25914534 Free PMC article. Review.
-
Bone health in type 1 diabetes: focus on evaluation and treatment in clinical practice.J Endocrinol Invest. 2015 Sep;38(9):941-50. doi: 10.1007/s40618-015-0284-9. Epub 2015 Apr 12. J Endocrinol Invest. 2015. PMID: 25863666 Review.
-
Identification of gene mutations associated with type 1 diabetes by next-generation sequencing in affected Palestinian families.Front Genet. 2024 Jan 11;14:1292073. doi: 10.3389/fgene.2023.1292073. eCollection 2023. Front Genet. 2024. PMID: 38274107 Free PMC article.
-
Tau and mTOR: The Hotspots for Multifarious Diseases in Alzheimer's Development.Front Neurosci. 2019 Jan 10;12:1017. doi: 10.3389/fnins.2018.01017. eCollection 2018. Front Neurosci. 2019. PMID: 30686983 Free PMC article. Review.
References
-
- Meyer HE, Tverdal A, Falch JA. Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol. 1993;137:1203–1211. - PubMed
-
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes care. 2001;24:1192–1197. - PubMed
-
- Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes care. 2006;29:1573–1578. - PubMed
-
- Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, et al. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001;12:811–822. - PubMed
-
- Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
