Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties
- PMID: 23530041
- PMCID: PMC3650378
- DOI: 10.1074/jbc.M112.428185
Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties
Abstract
Background: Myristate is a novel potential substrate for sphingoid base synthesis.
Results: Myocardial sphingoid base synthesis utilizes myristate; these sphingolipids are functionally non-redundant with canonical sphingoid bases.
Conclusion: d16:0 and d16:1 sphingolipids constitute an appreciable proportion of cardiac dihydrosphingosine and dihydroceramide, with distinct biological roles.
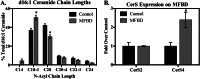
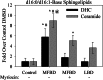
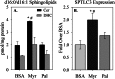
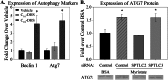
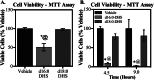
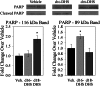
Significance: This pool of sphingolipids may play a heretofore unsuspected role in myocardial pathology or protection. The enzyme serine palmitoyltransferase (SPT) catalyzes the formation of the sphingoid base "backbone" from which all sphingolipids are derived. Previous studies have shown that inhibition of SPT ameliorates pathological cardiac outcomes in models of lipid overload, but the metabolites responsible for these phenotypes remain unidentified. Recent in vitro studies have shown that incorporation of the novel subunit SPTLC3 broadens the substrate specificity of SPT, allowing utilization of myristoyl-coenzyme A (CoA) in addition to its canonical substrate palmitoyl-CoA. However, the relevance of these findings in vivo has yet to be determined. The present study sought to determine whether myristate-derived d16 sphingolipids are represented among myocardial sphingolipids and, if so, whether their function and metabolic routes were distinct from those of palmitate-derived d18 sphingolipids. Data showed that d16:0 sphingoid bases occurred in more than one-third of total dihydrosphingosine and dihydroceramides in myocardium, and a diet high in saturated fat promoted their de novo production. Intriguingly, d16-ceramides demonstrated highly limited N-acyl chain diversity, and in vitro enzyme activity assays showed that these bases were utilized preferentially to canonical bases by CerS1. Functional differences between myristate- and palmitate-derived sphingolipids were observed in that, unlike d18 sphingolipids and SPTLC2, d16 sphingolipids and SPTLC3 did not appear to contribute to myristate-induced autophagy, whereas only d16 sphingolipids promoted cell death and cleavage of poly(ADP-ribose) polymerase in cardiomyocytes. Thus, these results reveal a previously unappreciated component of cardiac sphingolipids with functional differences from canonical sphingolipids.
Keywords: Cardiac Muscle; Cell Metabolism; Ceramide; Ceramide Synthase; Heart; Lipid Metabolism; Obesity; Serine Palmitoyltransferase; Sphingolipid.
Figures


Similar articles
-
The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases.J Biol Chem. 2009 Sep 25;284(39):26322-30. doi: 10.1074/jbc.M109.023192. Epub 2009 Aug 1. J Biol Chem. 2009. PMID: 19648650 Free PMC article.
-
SPTLC3 Is Essential for Complex I Activity and Contributes to Ischemic Cardiomyopathy.Circulation. 2024 Aug 20;150(8):622-641. doi: 10.1161/CIRCULATIONAHA.123.066879. Epub 2024 Apr 25. Circulation. 2024. PMID: 38660786
-
Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15591-15598. doi: 10.1073/pnas.2002391117. Epub 2020 Jun 23. Proc Natl Acad Sci U S A. 2020. PMID: 32576697 Free PMC article.
-
Serine Palmitoyltransferase Subunit 3 and Metabolic Diseases.Adv Exp Med Biol. 2022;1372:47-56. doi: 10.1007/978-981-19-0394-6_4. Adv Exp Med Biol. 2022. PMID: 35503173 Review.
-
Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption.Biochem Soc Trans. 2001 Nov;29(Pt 6):831-5. doi: 10.1042/0300-5127:0290831. Biochem Soc Trans. 2001. PMID: 11709083 Review.
Cited by
-
Structural insights into the assembly and substrate selectivity of human SPT-ORMDL3 complex.Nat Struct Mol Biol. 2021 Mar;28(3):249-257. doi: 10.1038/s41594-020-00553-7. Epub 2021 Feb 8. Nat Struct Mol Biol. 2021. PMID: 33558762
-
Expression of Ceramide Synthases in Mice and Their Roles in Regulating Acyl-Chain Sphingolipids: A Framework for Baseline Levels and Future Implications in Aging and Disease.Mol Pharmacol. 2024 Feb 15;105(3):131-143. doi: 10.1124/molpharm.123.000788. Mol Pharmacol. 2024. PMID: 38164625 Free PMC article.
-
Potential Drug _targets for Ceramide Metabolism in Cardiovascular Disease.J Cardiovasc Dev Dis. 2022 Dec 2;9(12):434. doi: 10.3390/jcdd9120434. J Cardiovasc Dev Dis. 2022. PMID: 36547431 Free PMC article. Review.
-
Sphingolipids in metabolic disease: The good, the bad, and the unknown.Cell Metab. 2021 Jul 6;33(7):1293-1306. doi: 10.1016/j.cmet.2021.06.006. Cell Metab. 2021. PMID: 34233172 Free PMC article. Review.
-
Sphingolipids in High Fat Diet and Obesity-Related Diseases.Mediators Inflamm. 2015;2015:520618. doi: 10.1155/2015/520618. Epub 2015 Nov 16. Mediators Inflamm. 2015. PMID: 26648664 Free PMC article. Review.
References
-
- Cowart L. A. (2009) Sphingolipids. Players in the pathology of metabolic disease. Trends Endocrinol. Metab. 20, 34–42 - PubMed
-
- Brice S. E., Cowart L. A. (2011) Sphingolipid metabolism and analysis in metabolic disease. Adv. Exp. Med. Biol. 721, 1–17 - PubMed
-
- Hanada K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

