Molecular control of the amount, subcellular location, and activity state of translation elongation factor 2 in neurons experiencing stress
- PMID: 23542375
- PMCID: PMC3772990
- DOI: 10.1016/j.freeradbiomed.2013.03.016
Molecular control of the amount, subcellular location, and activity state of translation elongation factor 2 in neurons experiencing stress
Abstract
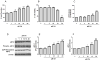
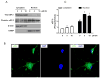
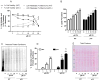
Eukaryotic elongation factor 2 (eEF-2) is an important regulator of the protein translation machinery whereby it controls the movement of the ribosome along the mRNA. The activity of eEF-2 is regulated by changes in cellular energy status and nutrient availability and by posttranslational modifications such as phosphorylation and mono-ADP-ribosylation. However, the mechanisms regulating protein translation under conditions of cellular stress in neurons are unknown. Here we show that when rat hippocampal neurons experience oxidative stress (lipid peroxidation induced by exposure to cumene hydroperoxide; CH), eEF-2 is hyperphosphorylated and ribosylated, resulting in reduced translational activity. The degradation of eEF-2 requires calpain proteolytic activity and is accompanied by accumulation of eEF-2 in the nuclear compartment. The subcellular localization of both native and phosphorylated forms of eEF-2 is influenced by CRM1 and 14.3.3, respectively. In hippocampal neurons p53 interacts with nonphosphorylated (active) eEF-2, but not with its phosphorylated form. The p53-eEF-2 complexes are present in cytoplasm and nucleus, and their abundance increases when neurons experience oxidative stress. The nuclear localization of active eEF-2 depends upon its interaction with p53, as cells lacking p53 contain less active eEF-2 in the nuclear compartment. Overexpression of eEF-2 in hippocampal neurons results in increased nuclear levels of eEF-2 and decreased cell death after exposure to CH. Our results reveal novel molecular mechanisms controlling the differential subcellular localization and activity state of eEF-2 that may influence the survival status of neurons during periods of elevated oxidative stress.
Keywords: 14.3.3; CRM1; Eukaryotic elongation factor 2; Free radicals; Hippocampal neurons; Lipid peroxidation; eEF-2; p53.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest; The authors declare no competing financial interests.
Figures
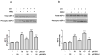

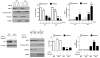


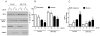
Similar articles
-
In vitro and in vivo protection by melatonin against the decline of elongation factor-2 caused by lipid peroxidation: preservation of protein synthesis.J Pineal Res. 2012 Aug;53(1):1-10. doi: 10.1111/j.1600-079X.2011.00961.x. Epub 2012 Mar 29. J Pineal Res. 2012. PMID: 22462727
-
"In vitro" effect of lipid peroxidation metabolites on elongation factor-2.Biochim Biophys Acta. 2006 Mar;1760(3):445-52. doi: 10.1016/j.bbagen.2005.12.019. Epub 2006 Jan 18. Biochim Biophys Acta. 2006. PMID: 16469450
-
Ribosomes terminated in vitro are in a tight association with non-phosphorylated elongation factor 2 (eEF-2) and GDP.Eur J Biochem. 1993 Jul 15;215(2):291-6. doi: 10.1111/j.1432-1033.1993.tb18034.x. Eur J Biochem. 1993. PMID: 8344297
-
Peptide-chain elongation in eukaryotes.Mol Biol Rep. 1994 May;19(3):161-70. doi: 10.1007/BF00986958. Mol Biol Rep. 1994. PMID: 7969104 Review.
-
Phosphorylation of elongation factor 2: a key mechanism regulating gene expression in vertebrates.New Biol. 1990 Oct;2(10):843-50. New Biol. 1990. PMID: 1964087 Review.
Cited by
-
Neuroproteomics and Systems Biology Approach to Identify Temporal Biomarker Changes Post Experimental Traumatic Brain Injury in Rats.Front Neurol. 2016 Nov 22;7:198. doi: 10.3389/fneur.2016.00198. eCollection 2016. Front Neurol. 2016. PMID: 27920753 Free PMC article.
-
Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal.Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. Epub 2014 May 8. Oxid Med Cell Longev. 2014. PMID: 24999379 Free PMC article. Review.
-
Interleukin-1β effect on the endogenous ADP-ribosylation and phosphorylation of eukaryotic elongation factor 2.Cytotechnology. 2016 Dec;68(6):2659-2666. doi: 10.1007/s10616-016-9990-1. Epub 2016 Aug 10. Cytotechnology. 2016. PMID: 27510652 Free PMC article.
-
Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain.Cell Death Dis. 2017 Jul 6;8(7):e2912. doi: 10.1038/cddis.2017.289. Cell Death Dis. 2017. PMID: 28682313 Free PMC article.
-
Elongation factor 2 diphthamide is critical for translation of two IRES-dependent protein _targets, XIAP and FGF2, under oxidative stress conditions.Free Radic Biol Med. 2014 Feb;67:131-8. doi: 10.1016/j.freeradbiomed.2013.10.015. Epub 2013 Oct 17. Free Radic Biol Med. 2014. PMID: 24140707 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

