Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection
- PMID: 23555812
- PMCID: PMC3612069
- DOI: 10.1371/journal.pone.0059863
Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection
Abstract
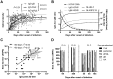
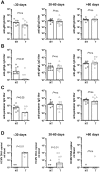
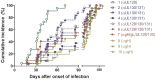
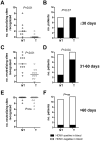
Primary human cytomegalovirus (HCMV) infections during pregnancy are associated with a high risk of virus transmission to the fetus. To identify correlates of intrauterine HCMV transmission, serial serum samples from HCMV transmitter and non-transmitter pregnant women with primary HCMV infection were analyzed for the presence of neutralizing antibodies against different glycoproteins and glycoprotein complexes, which are known to mediate entry into distinct types of host cells. Neutralizing activity was detected in the sera early after primary infection; absorption with a soluble pentameric complex formed by gH/gL/pUL128-131, but not with gH/gL dimer or with gB, abolished the capacity of sera to neutralize infection of epithelial cells. Importantly, an early, high antibody response to pentamer antigenic sites was associated with a significantly reduced risk of HCMV transmission to the fetus. This association is consistent with the high in vitro inhibition of HCMV infection of epithelial/endothelial cells as well as cell-to-cell spreading and virus transfer to leukocytes by anti-pentamer antibodies. Taken together, these findings indicate that the HCMV pentamer complex is a major _target of the antibody-mediated maternal immunity.
Conflict of interest statement
Figures



Similar articles
-
Antibodies against neutralization epitopes of human cytomegalovirus gH/gL/pUL128-130-131 complex and virus spreading may correlate with virus control in vivo.J Clin Immunol. 2012 Dec;32(6):1324-31. doi: 10.1007/s10875-012-9739-3. Epub 2012 Jul 27. J Clin Immunol. 2012. PMID: 22836657
-
Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation.J Virol. 2016 Jun 24;90(14):6216-6223. doi: 10.1128/JVI.00121-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122579 Free PMC article.
-
Soluble Human Cytomegalovirus gH/gL/pUL128-131 Pentameric Complex, but Not gH/gL, Inhibits Viral Entry to Epithelial Cells and Presents Dominant Native Neutralizing Epitopes.J Biol Chem. 2015 Jun 26;290(26):15985-95. doi: 10.1074/jbc.M115.652230. Epub 2015 May 6. J Biol Chem. 2015. PMID: 25947373 Free PMC article.
-
Role of human cytomegalovirus (HCMV)-specific antibody in HCMV-infected pregnant women.Early Hum Dev. 2014 Mar;90 Suppl 1:S32-4. doi: 10.1016/S0378-3782(14)70011-8. Early Hum Dev. 2014. PMID: 24709453 Review.
-
Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy.Rev Med Virol. 2017 Mar;27(2). doi: 10.1002/rmv.1921. Epub 2016 Dec 23. Rev Med Virol. 2017. PMID: 28008685 Review.
Cited by
-
Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children.Front Immunol. 2016 Jun 30;7:257. doi: 10.3389/fimmu.2016.00257. eCollection 2016. Front Immunol. 2016. PMID: 27446087 Free PMC article. Review.
-
Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy.Med Microbiol Immunol. 2015 Jun;204(3):263-71. doi: 10.1007/s00430-015-0399-9. Epub 2015 Mar 13. Med Microbiol Immunol. 2015. PMID: 25764180 Review.
-
Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection.Viruses. 2018 Aug 3;10(8):405. doi: 10.3390/v10080405. Viruses. 2018. PMID: 30081449 Free PMC article. Review.
-
Mathematical Modeling of Rhesus Cytomegalovirus Transplacental Transmission in Seronegative Rhesus Macaques.Viruses. 2023 Oct 1;15(10):2040. doi: 10.3390/v15102040. Viruses. 2023. PMID: 37896817 Free PMC article.
-
Recent Approaches and Strategies in the Generation of Anti-human Cytomegalovirus Vaccines.Methods Mol Biol. 2021;2244:403-463. doi: 10.1007/978-1-0716-1111-1_19. Methods Mol Biol. 2021. PMID: 33555597
References
-
- Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17: 253–276. - PubMed
-
- Revello MG, Fabbri E, Furione M, Zavattoni M, Lilleri D, et al. (2011) Role of prenatal diagnosis and counselling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection. A 20-year experience. J Clin Virol 50: 303–307. - PubMed
-
- Bodeus M, Hubinont C, Goubau P (1999) Increased risk of cytomegalovirus transmission in utero during late gestation. Obstet Gynecol 93: 658–660. - PubMed
-
- Dollard SC, Grosse SD, Ross DS (2007) New estimates of the prevalence of neurological sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17: 355–363. - PubMed
-
- Fowler KB, Boppana SB (2006) Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2: 226e31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

