Effect of alanine replacement of l17 and f19 on the aggregation and neurotoxicity of arctic-type aβ40
- PMID: 23634215
- PMCID: PMC3636269
- DOI: 10.1371/journal.pone.0061874
Effect of alanine replacement of l17 and f19 on the aggregation and neurotoxicity of arctic-type aβ40
Abstract
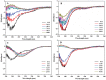
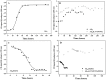
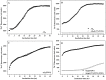
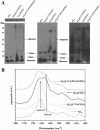
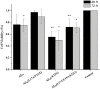
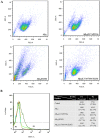
Alzheimer's disease is the most common form of neurodegenerative disease. Beta-amyloid peptides (Aβ) are responsible for neuronal death both in vitro and in vivo. Previously, L17 and F19 residues were identified as playing key roles in the stabilization of the Aβ40 conformation and in the reduction of its neurotoxicity. In this study, the effects of L17A/F19A mutations on the neurotoxicity of Aβ genetic mutant Arctic-type Aβ40(E22G) were tested. The results showed that compared to Aβ40(E22G), Aβ40(L17A/F19A/E22G) reduced the rate of conformation conversion, aggregation, and cytotoxicity, suggesting that L17 and F19 are critical residues responsible for conformational changes which may trigger the neurotoxic cascade of Aβ. Aβ40(L17A/F19A/E22G) also had decreased damage due to reactive oxygen species. The results are consistent with the discordant helix hypothesis, and confirm that residues 17-25 are in the discordant helix region. Compared to Aβ40(L17A/F19A), reduction in aggregation of Aβ40(L17A/F19A/E22G) was less significantly decreased. This observation provides an explanation based on the discordant helix hypothesis that the mutation of E22 to G22 of Aβ40(E22G) alters the propensity of the discordant helix. Arctic-type Aβ40(E22G) aggregates more severely than wild-type Aβ40, with a consequential increase in toxicity.
Conflict of interest statement
Figures

Similar articles
-
L17A/F19A Substitutions Augment the α-Helicity of β-Amyloid Peptide Discordant Segment.PLoS One. 2016 Apr 22;11(4):e0154327. doi: 10.1371/journal.pone.0154327. eCollection 2016. PLoS One. 2016. PMID: 27104649 Free PMC article.
-
Conformational Characterization of Native and L17A/F19A-Substituted Dutch-Type β-Amyloid Peptides.Int J Mol Sci. 2020 Apr 7;21(7):2571. doi: 10.3390/ijms21072571. Int J Mol Sci. 2020. PMID: 32272787 Free PMC article.
-
Aβ40(L17A/F19A) mutant diminishes the aggregation and neurotoxicity of Aβ40.Biochem Biophys Res Commun. 2011 Feb 4;405(1):91-5. doi: 10.1016/j.bbrc.2010.12.133. Epub 2011 Jan 7. Biochem Biophys Res Commun. 2011. PMID: 21216230
-
Molecular insights into the effect L17A/F19A double mutation on the structure and dynamics of Aβ40 : A molecular dynamics simulation study.J Cell Biochem. 2018 Nov;119(11):8949-8961. doi: 10.1002/jcb.27149. Epub 2018 Aug 4. J Cell Biochem. 2018. PMID: 30076733
-
Impact of a discordant helix on β-amyloid structure, aggregation ability and toxicity.Eur Biophys J. 2017 Oct;46(7):681-687. doi: 10.1007/s00249-017-1235-5. Epub 2017 Jul 7. Eur Biophys J. 2017. PMID: 28687859 Review.
Cited by
-
Impact of Mutations on the Conformational Transition from α-Helix to β-Sheet Structures in Arctic-Type Aβ40: Insights from Molecular Dynamics Simulations.ACS Omega. 2020 Aug 28;5(36):23219-23228. doi: 10.1021/acsomega.0c02983. eCollection 2020 Sep 15. ACS Omega. 2020. PMID: 32954172 Free PMC article.
-
Effect of C-terminal residues of Aβ on copper binding affinity, structural conversion and aggregation.PLoS One. 2014 Mar 3;9(3):e90385. doi: 10.1371/journal.pone.0090385. eCollection 2014. PLoS One. 2014. PMID: 24594588 Free PMC article.
-
L17A/F19A Substitutions Augment the α-Helicity of β-Amyloid Peptide Discordant Segment.PLoS One. 2016 Apr 22;11(4):e0154327. doi: 10.1371/journal.pone.0154327. eCollection 2016. PLoS One. 2016. PMID: 27104649 Free PMC article.
-
Protein folding, misfolding and aggregation: The importance of two-electron stabilizing interactions.PLoS One. 2017 Sep 18;12(9):e0180905. doi: 10.1371/journal.pone.0180905. eCollection 2017. PLoS One. 2017. PMID: 28922400 Free PMC article.
-
Conformational Characterization of Native and L17A/F19A-Substituted Dutch-Type β-Amyloid Peptides.Int J Mol Sci. 2020 Apr 7;21(7):2571. doi: 10.3390/ijms21072571. Int J Mol Sci. 2020. PMID: 32272787 Free PMC article.
References
-
- Selkoe DJ (1991) Amyloid protein and Alzheimer’s disease. Scientific American 265: 68–71, 74–66, 78. - PubMed
-
- Holtzman DM, Mobley WC (1991) Molecular studies in Alzheimer’s disease. Trends in biochemical sciences 16: 140–144. - PubMed
-
- Chui HC, Teng EL, Henderson VW, Moy AC (1985) Clinical subtypes of dementia of the Alzheimer type. Neurology 35: 1544–1550. - PubMed
-
- Mucke L (2009) Neuroscience: Alzheimer’s disease. Nature 461: 895–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

