Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation
- PMID: 23650383
- PMCID: PMC3666735
- DOI: 10.1073/pnas.1221294110
Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation
Abstract
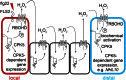
In animals and plants, pathogen recognition triggers the local activation of intracellular signaling that is prerequisite for mounting systemic defenses in the whole organism. We identified that Arabidopsis thaliana isoform CPK5 of the plant calcium-dependent protein kinase family becomes rapidly biochemically activated in response to pathogen-associated molecular pattern (PAMP) stimulation. CPK5 signaling resulted in enhanced salicylic acid-mediated resistance to the bacterial pathogen Pst DC3000, differential plant defense gene expression, and synthesis of reactive oxygen species (ROS). Using selected reaction monitoring MS, we identified the plant NADPH oxidase, respiratory burst oxidase homolog D (RBOHD), as an in vivo phosphorylation _target of CPK5. Remarkably, CPK5-dependent in vivo phosphorylation of RBOHD occurs on both PAMP- and ROS stimulation. Furthermore, rapid CPK5-dependent biochemical and transcriptional activation of defense reactions at distal sites is compromised in cpk5 and rbohd mutants. Our data not only identify CPK5 as a key regulator of innate immune responses in plants but also support a model of ROS-mediated cell-to-cell communication, where a self-propagating mutual activation circuit consisting of the protein kinase, CPK5, and the NADPH oxidase RBOHD facilitates rapid signal propagation as a prerequisite for defense response activation at distal sites within the plant.
Keywords: ROS signaling; disease resistance; plant innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity.Mol Cell. 2014 Apr 10;54(1):43-55. doi: 10.1016/j.molcel.2014.02.021. Epub 2014 Mar 12. Mol Cell. 2014. PMID: 24630626
-
Regulation of the NADPH Oxidase RBOHD During Plant Immunity.Plant Cell Physiol. 2015 Aug;56(8):1472-80. doi: 10.1093/pcp/pcv063. Epub 2015 May 4. Plant Cell Physiol. 2015. PMID: 25941234 Review.
-
CALCIUM-DEPENDENT PROTEIN KINASE5 Associates with the Truncated NLR Protein TIR-NBS2 to Contribute to exo70B1-Mediated Immunity.Plant Cell. 2017 Apr;29(4):746-759. doi: 10.1105/tpc.16.00822. Epub 2017 Mar 28. Plant Cell. 2017. PMID: 28351987 Free PMC article.
-
CRK2 and C-terminal Phosphorylation of NADPH Oxidase RBOHD Regulate Reactive Oxygen Species Production in Arabidopsis.Plant Cell. 2020 Apr;32(4):1063-1080. doi: 10.1105/tpc.19.00525. Epub 2020 Feb 7. Plant Cell. 2020. PMID: 32034035 Free PMC article.
-
Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD.Plant Cell Rep. 2016 May;35(5):995-1007. doi: 10.1007/s00299-016-1950-x. Epub 2016 Feb 16. Plant Cell Rep. 2016. PMID: 26883222 Review.
Cited by
-
Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells.Elife. 2015 Jul 20;4:e03599. doi: 10.7554/eLife.03599. Elife. 2015. PMID: 26192964 Free PMC article.
-
The Fundamental Role of NOX Family Proteins in Plant Immunity and Their Regulation.Int J Mol Sci. 2016 May 27;17(6):805. doi: 10.3390/ijms17060805. Int J Mol Sci. 2016. PMID: 27240354 Free PMC article. Review.
-
Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination.Plant Physiol. 2015 Apr;167(4):1630-42. doi: 10.1104/pp.114.251298. Epub 2015 Feb 13. Plant Physiol. 2015. PMID: 25681329 Free PMC article.
-
ROS Regulation of Polar Growth in Plant Cells.Plant Physiol. 2016 Jul;171(3):1593-605. doi: 10.1104/pp.16.00191. Epub 2016 May 4. Plant Physiol. 2016. PMID: 27208283 Free PMC article. Review.
-
The interaction of ABA and ROS in plant growth and stress resistances.Front Plant Sci. 2022 Nov 24;13:1050132. doi: 10.3389/fpls.2022.1050132. eCollection 2022. Front Plant Sci. 2022. PMID: 36507454 Free PMC article. Review.
References
-
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. - PubMed
-
- Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. - PubMed
-
- Schwessinger B, Zipfel C. News from the frontline: Recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol. 2008;11(4):389–395. - PubMed
-
- Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005;17(3):385–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

