Bi-parental care contributes to sexually dimorphic neural cell genesis in the adult mammalian brain
- PMID: 23650527
- PMCID: PMC3641101
- DOI: 10.1371/journal.pone.0062701
Bi-parental care contributes to sexually dimorphic neural cell genesis in the adult mammalian brain
Abstract
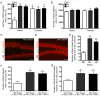
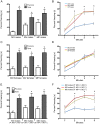
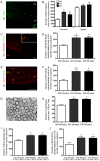
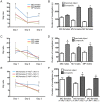
Early life events can modulate brain development to produce persistent physiological and behavioural phenotypes that are transmissible across generations. However, whether neural precursor cells are altered by early life events, to produce persistent and transmissible behavioural changes, is unknown. Here, we show that bi-parental care, in early life, increases neural cell genesis in the adult rodent brain in a sexually dimorphic manner. Bi-parentally raised male mice display enhanced adult dentate gyrus neurogenesis, which improves hippocampal neurogenesis-dependent learning and memory. Female mice display enhanced adult white matter oligodendrocyte production, which increases proficiency in bilateral motor coordination and preference for social investigation. Surprisingly, single parent-raised male and female offspring, whose fathers and mothers received bi-parental care, respectively, display a similar enhancement in adult neural cell genesis and phenotypic behaviour. Therefore, neural plasticity and behavioural effects due to bi-parental care persist throughout life and are transmitted to the next generation.
Conflict of interest statement
Figures
Similar articles
-
Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation.Nature. 2011 Apr 28;472(7344):466-70. doi: 10.1038/nature09817. Epub 2011 Apr 3. Nature. 2011. PMID: 21460835 Free PMC article.
-
Adult neurogenesis in the mammalian dentate gyrus.Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
-
Maturation Dynamics of the Axon Initial Segment (AIS) of Newborn Dentate Granule Cells in Young Adult C57BL/6J Mice.J Neurosci. 2019 Feb 27;39(9):1605-1620. doi: 10.1523/JNEUROSCI.2253-18.2019. Epub 2019 Jan 16. J Neurosci. 2019. PMID: 30651327 Free PMC article.
-
Diversity of Neural Precursors in the Adult Mammalian Brain.Cold Spring Harb Perspect Biol. 2016 Apr 1;8(4):a018838. doi: 10.1101/cshperspect.a018838. Cold Spring Harb Perspect Biol. 2016. PMID: 26988967 Free PMC article. Review.
-
Developmental cuprizone exposure impairs oligodendrocyte lineages differentially in cortical and white matter tissues and suppresses glutamatergic neurogenesis signals and synaptic plasticity in the hippocampal dentate gyrus of rats.Toxicol Appl Pharmacol. 2016 Jan 1;290:10-20. doi: 10.1016/j.taap.2015.11.006. Epub 2015 Nov 11. Toxicol Appl Pharmacol. 2016. PMID: 26577399
Cited by
-
Fathering in rodents: Neurobiological substrates and consequences for offspring.Horm Behav. 2016 Jan;77:249-59. doi: 10.1016/j.yhbeh.2015.05.021. Epub 2015 Jun 26. Horm Behav. 2016. PMID: 26122293 Free PMC article. Review.
-
In the pursuit of new social neurons. Neurogenesis and social behavior in mice: A systematic review.Front Cell Dev Biol. 2022 Nov 4;10:1011657. doi: 10.3389/fcell.2022.1011657. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36407114 Free PMC article. Review.
-
Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition?Cell Mol Life Sci. 2014 Jul;71(13):2499-515. doi: 10.1007/s00018-014-1568-5. Epub 2014 Feb 13. Cell Mol Life Sci. 2014. PMID: 24522255 Free PMC article. Review.
References
-
- Branchi I (2009) The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev 33: 551–559. - PubMed
-
- Champagne FA (2010) Epigenetic influence of social experiences across the lifespan. Dev Psychobiol 52: 299–311. - PubMed
-
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, et al. (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662. - PubMed
-
- Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161–1192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

