Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity
- PMID: 23671105
- PMCID: PMC3670398
- DOI: 10.1073/pnas.1219451110
Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity
Abstract
Obesity and type 2 diabetes are characterized by altered gut microbiota, inflammation, and gut barrier disruption. Microbial composition and the mechanisms of interaction with the host that affect gut barrier function during obesity and type 2 diabetes have not been elucidated. We recently isolated Akkermansia muciniphila, which is a mucin-degrading bacterium that resides in the mucus layer. The presence of this bacterium inversely correlates with body weight in rodents and humans. However, the precise physiological roles played by this bacterium during obesity and metabolic disorders are unknown. This study demonstrated that the abundance of A. muciniphila decreased in obese and type 2 diabetic mice. We also observed that prebiotic feeding normalized A. muciniphila abundance, which correlated with an improved metabolic profile. In addition, we demonstrated that A. muciniphila treatment reversed high-fat diet-induced metabolic disorders, including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance. A. muciniphila administration increased the intestinal levels of endocannabinoids that control inflammation, the gut barrier, and gut peptide secretion. Finally, we demonstrated that all these effects required viable A. muciniphila because treatment with heat-killed cells did not improve the metabolic profile or the mucus layer thickness. In summary, this study provides substantial insight into the intricate mechanisms of bacterial (i.e., A. muciniphila) regulation of the cross-talk between the host and gut microbiota. These results also provide a rationale for the development of a treatment that uses this human mucus colonizer for the prevention or treatment of obesity and its associated metabolic disorders.
Keywords: LPS; Lactobacillus plantarum; RegIIIγ; antimicrobial peptides; gut permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

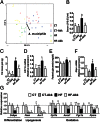
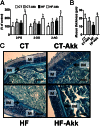

Comment in
-
Microbiome: A mucus colonizer manages host metabolism.Nat Rev Microbiol. 2013 Jul;11(7):430-1. doi: 10.1038/nrmicro3051. Epub 2013 May 28. Nat Rev Microbiol. 2013. PMID: 23712351 Free PMC article. No abstract available.
Similar articles
-
Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr-/-.Leiden Mice.Int J Mol Sci. 2022 Feb 19;23(4):2325. doi: 10.3390/ijms23042325. Int J Mol Sci. 2022. PMID: 35216439 Free PMC article.
-
Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders.Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):8075-8083. doi: 10.26355/eurrev_201909_19024. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599433 Review.
-
Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice.Sci Rep. 2015 Nov 13;5:16643. doi: 10.1038/srep16643. Sci Rep. 2015. PMID: 26563823 Free PMC article.
-
Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions.Exp Mol Med. 2018 Feb 23;50(2):e450. doi: 10.1038/emm.2017.282. Exp Mol Med. 2018. PMID: 29472701 Free PMC article.
-
Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders.Minerva Endocrinol (Torino). 2022 Jun;47(2):242-252. doi: 10.23736/S2724-6507.22.03752-6. Epub 2022 Feb 1. Minerva Endocrinol (Torino). 2022. PMID: 35103461 Review.
Cited by
-
Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes.Nutrients. 2024 Oct 11;16(20):3447. doi: 10.3390/nu16203447. Nutrients. 2024. PMID: 39458444 Free PMC article. Review.
-
The role of gut microbiota in cancer treatment: friend or foe?Gut. 2020 Oct;69(10):1867-1876. doi: 10.1136/gutjnl-2020-321153. Epub 2020 Aug 5. Gut. 2020. PMID: 32759302 Free PMC article. Review.
-
Lead exposure exacerbates adverse effects of HFD on metabolic function via disruption of gut microbiome, leading to compromised barrier function and inflammation.Eur J Nutr. 2023 Mar;62(2):783-795. doi: 10.1007/s00394-022-03028-1. Epub 2022 Oct 20. Eur J Nutr. 2023. PMID: 36264385
-
The PPARα Regulation of the Gut Physiology in Regard to Interaction with Microbiota, Intestinal Immunity, Metabolism, and Permeability.Int J Mol Sci. 2022 Nov 16;23(22):14156. doi: 10.3390/ijms232214156. Int J Mol Sci. 2022. PMID: 36430628 Free PMC article. Review.
-
Lipid and energy metabolism in Wilson disease.Liver Res. 2020 Mar;4(1):5-14. doi: 10.1016/j.livres.2020.02.002. Epub 2020 Feb 19. Liver Res. 2020. PMID: 32832193 Free PMC article.
References
-
- Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. - PubMed
-
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. - PubMed
-
- Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

