Coronary microvascular pericytes are the cellular _target of sunitinib malate-induced cardiotoxicity
- PMID: 23720580
- PMCID: PMC3833098
- DOI: 10.1126/scitranslmed.3005066
Coronary microvascular pericytes are the cellular _target of sunitinib malate-induced cardiotoxicity
Abstract
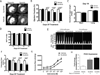
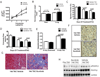
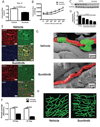
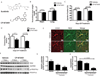
Sunitinib malate is a multi_targeted receptor tyrosine kinase inhibitor used in the treatment of human malignancies. A substantial number of sunitinib-treated patients develop cardiac dysfunction, but the mechanism of sunitinib-induced cardiotoxicity is poorly understood. We show that mice treated with sunitinib develop cardiac and coronary microvascular dysfunction and exhibit an impaired cardiac response to stress. The physiological changes caused by treatment with sunitinib are accompanied by a substantial depletion of coronary microvascular pericytes. Pericytes are a cell type that is dependent on intact platelet-derived growth factor receptor (PDGFR) signaling but whose role in the heart is poorly defined. Sunitinib-induced pericyte depletion and coronary microvascular dysfunction are recapitulated by CP-673451, a structurally distinct PDGFR inhibitor, confirming the role of PDGFR in pericyte survival. Thalidomide, an anticancer agent that is known to exert beneficial effects on pericyte survival and function, prevents sunitinib-induced pericyte cell death in vitro and prevents sunitinib-induced cardiotoxicity in vivo in a mouse model. Our findings suggest that pericytes are the primary cellular _target of sunitinib-induced cardiotoxicity and reveal the pericyte as a cell type of concern in the regulation of coronary microvascular function. Furthermore, our data provide preliminary evidence that thalidomide may prevent cardiotoxicity in sunitinib-treated cancer patients.
Figures

Comment in
-
Cardio-oncology: it takes two to translate.Sci Transl Med. 2013 May 29;5(187):187fs20. doi: 10.1126/scitranslmed.3006490. Sci Transl Med. 2013. PMID: 23720578 No abstract available.
Similar articles
-
Cardio-oncology: it takes two to translate.Sci Transl Med. 2013 May 29;5(187):187fs20. doi: 10.1126/scitranslmed.3006490. Sci Transl Med. 2013. PMID: 23720578 No abstract available.
-
Sirt3 promotes sensitivity to sunitinib-induced cardiotoxicity via inhibition of GTSP1/JNK/autophagy pathway in vivo and in vitro.Arch Toxicol. 2019 Nov;93(11):3249-3260. doi: 10.1007/s00204-019-02573-9. Epub 2019 Sep 24. Arch Toxicol. 2019. PMID: 31552474
-
Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study.Mol Cancer Ther. 2010 Jun;9(6):1525-35. doi: 10.1158/1535-7163.MCT-09-1106. Epub 2010 May 25. Mol Cancer Ther. 2010. PMID: 20501804 Free PMC article.
-
A preclinical review of sunitinib, a multi_targeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities.Ann Oncol. 2007 Sep;18 Suppl 10:x3-10. doi: 10.1093/annonc/mdm408. Ann Oncol. 2007. PMID: 17761721 Review.
-
Sunitinib: a multi_targeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies.BioDrugs. 2009;23(6):377-89. doi: 10.2165/11318860-000000000-00000. BioDrugs. 2009. PMID: 19894779 Review.
Cited by
-
Emerging Role of Pericytes and Their Secretome in the Heart.Cells. 2021 Mar 4;10(3):548. doi: 10.3390/cells10030548. Cells. 2021. PMID: 33806335 Free PMC article. Review.
-
Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1059-H1068. doi: 10.1152/ajpheart.00681.2020. Epub 2020 Oct 9. Am J Physiol Heart Circ Physiol. 2020. PMID: 33036546 Free PMC article. Review.
-
In vitro models of molecular and nano-particle transport across the blood-brain barrier.Biomicrofluidics. 2018 May 31;12(4):042213. doi: 10.1063/1.5027118. eCollection 2018 Jul. Biomicrofluidics. 2018. PMID: 29887937 Free PMC article.
-
Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells.Nat Protoc. 2014;9(6):1514-31. doi: 10.1038/nprot.2014.102. Epub 2014 May 29. Nat Protoc. 2014. PMID: 24874816
-
Physiological, pharmacological and toxicological considerations of drug-induced structural cardiac injury.Br J Pharmacol. 2015 Feb;172(4):957-74. doi: 10.1111/bph.12979. Epub 2015 Jan 12. Br J Pharmacol. 2015. PMID: 25302413 Free PMC article. Review.
References
-
- Di Lorenzo G, Autorino R, Bruni G, Carteni G, Ricevuto E, Tudini M, Ficorella C, Romano C, Aieta M, Giordano A, Giuliano M, Gonnella A, De Nunzio C, Rizzo M, Montesarchio V, Ewer M, De Placido S. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Annals of Oncology. 2009 Sep;20:1535. - PubMed
-
- Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Nov 10;26:5204. - PubMed
-
- Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, 2nd, Trent J, Champion JC, Durand JB, Lenihan DJ. Heart failure associated with sunitinib malate: a multi_targeted receptor tyrosine kinase inhibitor. Cancer. 2008 Jun;112:2500. - PubMed
-
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007 Dec 15;370:2011. - PMC - PubMed
-
- Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, Choueiri TK. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Sep 1;29:3450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

