11β-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects
- PMID: 23722901
- PMCID: PMC3696573
- DOI: 10.1172/JCI64162
11β-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects
Abstract
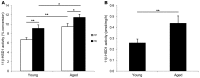
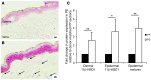
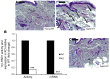
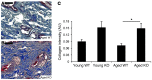
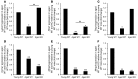
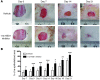
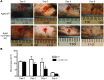
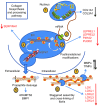
Glucocorticoid (GC) excess adversely affects skin integrity, inducing thinning and impaired wound healing. Aged skin, particularly that which has been photo-exposed, shares a similar phenotype. Previously, we demonstrated age-induced expression of the GC-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in cultured human dermal fibroblasts (HDFs). Here, we determined 11β-HSD1 levels in human skin biopsies from young and older volunteers and examined the aged 11β-HSD1 KO mouse skin phenotype. 11β-HSD1 activity was elevated in aged human and mouse skin and in PE compared with donor-matched photo-protected human biopsies. Age-induced dermal atrophy with deranged collagen structural organization was prevented in 11β-HSD1 KO mice, which also exhibited increased collagen density. We found that treatment of HDFs with physiological concentrations of cortisol inhibited rate-limiting steps in collagen biosynthesis and processing. Furthermore, topical 11β-HSD1 inhibitor treatment accelerated healing of full-thickness mouse dorsal wounds, with improved healing also observed in aged 11β-HSD1 KO mice. These findings suggest that elevated 11β-HSD1 activity in aging skin leads to increased local GC generation, which may account for adverse changes occurring in the elderly, and 11β-HSD1 inhibitors may be useful in the treatment of age-associated impairments in dermal integrity and wound healing.
Figures
Similar articles
-
Topical 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition Corrects Cutaneous Features of Systemic Glucocorticoid Excess in Female Mice.Endocrinology. 2018 Jan 1;159(1):547-556. doi: 10.1210/en.2017-00607. Endocrinology. 2018. PMID: 29087473 Free PMC article.
-
11β-hydroxysteroid dehydrogenase 1 specific inhibitor increased dermal collagen content and promotes fibroblast proliferation.PLoS One. 2014 Mar 25;9(3):e93051. doi: 10.1371/journal.pone.0093051. eCollection 2014. PLoS One. 2014. PMID: 24667799 Free PMC article.
-
Oral 11β-HSD1 inhibitor AZD4017 improves wound healing and skin integrity in adults with type 2 diabetes mellitus: a pilot randomized controlled trial.Eur J Endocrinol. 2022 Feb 28;186(4):441-455. doi: 10.1530/EJE-21-1197. Eur J Endocrinol. 2022. PMID: 35113805 Free PMC article. Clinical Trial.
-
Role of corticosteroids in skin physiology and therapeutic potential of an 11β-HSD1 inhibitor: A review.Int J Dermatol. 2024 Apr;63(4):443-454. doi: 10.1111/ijd.16967. Epub 2023 Dec 25. Int J Dermatol. 2024. PMID: 38146184 Review.
-
Inhibitors of 11β-hydroxysteroid dehydrogenase type 1 in antidiabetic therapy.Handb Exp Pharmacol. 2011;(203):127-46. doi: 10.1007/978-3-642-17214-4_6. Handb Exp Pharmacol. 2011. PMID: 21484570 Review.
Cited by
-
Increased Expression of 11β-Hydroxysteroid Dehydrogenase Type 1 Contributes to Epidermal Permeability Barrier Dysfunction in Aged Skin.Int J Mol Sci. 2021 May 27;22(11):5750. doi: 10.3390/ijms22115750. Int J Mol Sci. 2021. PMID: 34072239 Free PMC article.
-
Effect of AZD4017, a Selective 11β-HSD1 Inhibitor, on Bone Turnover Markers in Postmenopausal Osteopenia.J Clin Endocrinol Metab. 2022 Jun 16;107(7):2026-2035. doi: 10.1210/clinem/dgac100. J Clin Endocrinol Metab. 2022. PMID: 35275196 Free PMC article. Clinical Trial.
-
Characterization of Transcriptomic and Proteomic Changes in the Skin after Chronic Fluocinolone Acetonide Treatment.Biomolecules. 2022 Dec 6;12(12):1822. doi: 10.3390/biom12121822. Biomolecules. 2022. PMID: 36551249 Free PMC article.
-
Skin 11β-hydroxysteroid dehydrogenase type 1 enzyme expression regulates burn wound healing and can be _targeted to modify scar characteristics.Burns Trauma. 2023 Jan 20;11:tkac052. doi: 10.1093/burnst/tkac052. eCollection 2023. Burns Trauma. 2023. PMID: 36694861 Free PMC article.
-
Roles of the Glucocorticoid and Mineralocorticoid Receptors in Skin Pathophysiology.Int J Mol Sci. 2018 Jun 29;19(7):1906. doi: 10.3390/ijms19071906. Int J Mol Sci. 2018. PMID: 29966221 Free PMC article. Review.
References
-
- Korting HC, Unholzer A, Schafer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002;15(2):85–91. - PubMed
-
- Sowers JR, Lippman HR. Cushing’s syndrome due to ectopic ACTH production: cutaneous manifestations. Cutis. 1985;36(4):351–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

