Sirtuin-7 inhibits the activity of hypoxia-inducible factors
- PMID: 23750001
- PMCID: PMC3774348
- DOI: 10.1074/jbc.M113.476903
Sirtuin-7 inhibits the activity of hypoxia-inducible factors
Abstract
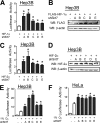
Hypoxia-inducible factor (HIF) 1 and HIF-2 are heterodimeric proteins composed of an oxygen-regulated HIF-1α or HIF-2α subunit, respectively, and a constitutively expressed HIF-1β subunit, which mediate adaptive transcriptional responses to hypoxia. Here, we report that Sirt7 (sirtuin-7) negatively regulates HIF-1α and HIF-2α protein levels by a mechanism that is independent of prolyl hydroxylation and that does not involve proteasomal or lysosomal degradation. The effect of Sirt7 was maintained in the presence of the sirtuin inhibitor nicotinamide and upon deletion or mutation of its deacetylase domain, indicating a non-catalytic function. Knockdown of Sirt7 led to an increase in HIF-1α and HIF-2α protein levels and an increase in HIF-1 and HIF-2 transcriptional activity. Thus, we identify a novel molecular function of Sirt7 as a negative regulator of HIF signaling.
Keywords: Hypoxia; Hypoxia-inducible Factor (HIF); Sirtuins; Transcription.
Figures

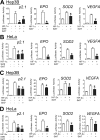
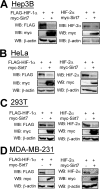
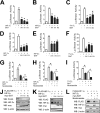
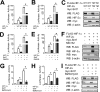
Similar articles
-
Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha.J Biol Chem. 2010 Feb 5;285(6):3651-3663. doi: 10.1074/jbc.M109.068577. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940151 Free PMC article.
-
HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc.J Bone Miner Res. 2012 Feb;27(2):401-12. doi: 10.1002/jbmr.538. J Bone Miner Res. 2012. PMID: 21987385 Free PMC article.
-
Lenticular cytoprotection. Part 1: the role of hypoxia inducible factors-1α and -2α and vascular endothelial growth factor in lens epithelial cell survival in hypoxia.Mol Vis. 2013;19:1-15. Epub 2013 Jan 2. Mol Vis. 2013. PMID: 23335846 Free PMC article.
-
Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review).Mol Med Rep. 2015 Aug;12(2):2411-6. doi: 10.3892/mmr.2015.3689. Epub 2015 Apr 27. Mol Med Rep. 2015. PMID: 25936862 Review.
-
Regulation of gene expression by the hypoxia-inducible factors.Mol Interv. 2002 Jul;2(4):229-43. doi: 10.1124/mi.2.4.229. Mol Interv. 2002. PMID: 14993394 Review.
Cited by
-
Role of Hypoxia and Metabolism in the Development of Neointimal Hyperplasia in Arteriovenous Fistulas.Int J Mol Sci. 2019 Oct 29;20(21):5387. doi: 10.3390/ijms20215387. Int J Mol Sci. 2019. PMID: 31671790 Free PMC article. Review.
-
Metabolic Alterations at the Crossroad of Aging and Oncogenesis.Int Rev Cell Mol Biol. 2017;332:1-42. doi: 10.1016/bs.ircmb.2017.01.003. Epub 2017 Feb 28. Int Rev Cell Mol Biol. 2017. PMID: 28526131 Free PMC article. Review.
-
Mitochondrial Reactive Oxygen Species and Kidney Hypoxia in the Development of Diabetic Nephropathy.Front Physiol. 2017 Apr 11;8:211. doi: 10.3389/fphys.2017.00211. eCollection 2017. Front Physiol. 2017. PMID: 28443030 Free PMC article. Review.
-
Sirtuins and Hypoxia in EMT Control.Pharmaceuticals (Basel). 2022 Jun 10;15(6):737. doi: 10.3390/ph15060737. Pharmaceuticals (Basel). 2022. PMID: 35745656 Free PMC article. Review.
-
Autophagy manipulation as a strategy for efficient anticancer therapies: possible consequences.J Exp Clin Cancer Res. 2019 Jun 14;38(1):262. doi: 10.1186/s13046-019-1275-z. J Exp Clin Cancer Res. 2019. PMID: 31200739 Free PMC article. Review.
References
-
- Yu A. Y., Shimoda L. A., Iyer N. V., Huso D. L., Sun X., McWilliams R., Beaty T., Sham J. S., Wiener C. M., Sylvester J. T., Semenza G. L. (1999) Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Invest. 103, 691–696 - PMC - PubMed
-
- Semenza G. L. (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24, 97–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

