Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope
- PMID: 23804643
- PMCID: PMC3754086
- DOI: 10.1128/JVI.01167-13
Comparative analysis of gO isoforms reveals that strains of human cytomegalovirus differ in the ratio of gH/gL/gO and gH/gL/UL128-131 in the virion envelope
Abstract
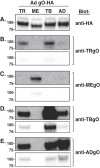
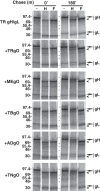
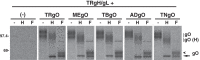
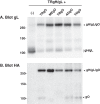
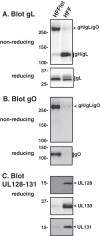
Herpesvirus glycoprotein complex gH/gL provides a core entry function through interactions with the fusion protein gB and can also influence tropism through receptor interactions. The Epstein-Barr virus gH/gL and gH/gL/gp42 serve both functions for entry into epithelial and B cells, respectively. Human cytomegalovirus (HCMV) gH/gL can be bound by the UL128-131 proteins or gO. The phenotypes of gO and UL128-131 mutants suggest that gO-gH/gL interactions are necessary for the core entry function on all cell types, whereas the binding of UL128-131 to gH/gL likely relates to a distinct receptor-binding function for entry into some specific cell types (e.g., epithelial) but not others (e.g., fibroblasts and neurons). There are at least eight isoforms of gO that differ by 10 to 30% of amino acids, and previous analysis of two HCMV strains suggested that some isoforms of gO function like chaperones, disassociating during assembly to leave unbound gH/gL in the virion envelope, while others remain bound to gH/gL. For the current report, we analyzed the gH/gL complexes present in the virion envelope of several HCMV strains, each of which encodes a distinct gO isoform. Results indicate that all strains of HCMV contain stable gH/gL/gO trimers and gH/gL/UL128-131 pentamers and little, if any, unbound gH/gL. TR, TB40/e, AD169, and PH virions contained vastly more gH/gL/gO than gH/gL/UL128-131, whereas Merlin virions contained mostly gH/gL/UL128-131, despite abundant unbound gO remaining in the infected cells. Suppression of UL128-131 expression during Merlin replication dramatically shifted the ratio toward gH/gL/gO. These data suggest that Merlin gO is less efficient than other gO isoforms at competing with UL128-131 for binding to gH/gL. Thus, gO diversity may influence the pathogenesis of HCMV through effects on the assembly of the core versus tropism gH/gL complexes.
Figures


Similar articles
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
Expression Levels of Glycoprotein O (gO) Vary between Strains of Human Cytomegalovirus, Influencing the Assembly of gH/gL Complexes and Virion Infectivity.J Virol. 2018 Jul 17;92(15):e00606-18. doi: 10.1128/JVI.00606-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743375 Free PMC article.
-
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
Cited by
-
Soluble Human Cytomegalovirus gH/gL/pUL128-131 Pentameric Complex, but Not gH/gL, Inhibits Viral Entry to Epithelial Cells and Presents Dominant Native Neutralizing Epitopes.J Biol Chem. 2015 Jun 26;290(26):15985-95. doi: 10.1074/jbc.M115.652230. Epub 2015 May 6. J Biol Chem. 2015. PMID: 25947373 Free PMC article.
-
Distribution of the CMV glycoprotein gH/gL/gO and gH/gL/pUL128/pUL130/pUL131A complex variants and associated clinical manifestations in infants infected congenitally or postnatally.Sci Rep. 2019 Nov 8;9(1):16352. doi: 10.1038/s41598-019-52906-y. Sci Rep. 2019. PMID: 31705022 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Intermittent bulk release of human cytomegalovirus.PLoS Pathog. 2022 Aug 4;18(8):e1010575. doi: 10.1371/journal.ppat.1010575. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35925870 Free PMC article.
-
Human cytomegalovirus infection of langerhans-type dendritic cells does not require the presence of the gH/gL/UL128-131A complex and is blocked after nuclear deposition of viral genomes in immature cells.J Virol. 2014 Jan;88(1):403-16. doi: 10.1128/JVI.03062-13. Epub 2013 Oct 23. J Virol. 2014. PMID: 24155395 Free PMC article.
References
-
- Britt WJ. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417–470 - PubMed
-
- Landolfo S, Gariglio M, Gribaudo G, Lembo D. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269–297 - PubMed
-
- Pass R, Fowler K, Boppana S, Britt W, Stagno S. 2006. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J. Clin. Virol. 35:216–220 - PubMed
-
- Plachter B, Sinzger C, Jahn G. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46:195–261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

