Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice
- PMID: 23826298
- PMCID: PMC3691251
- DOI: 10.1371/journal.pone.0067422
Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice
Abstract
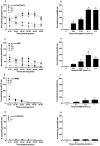
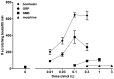
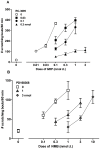
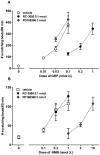
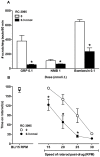
Pruritus (itch) is a severe side effect associated with the use of drugs as well as hepatic and hematological disorders. Previous studies in rodents suggest that bombesin receptor subtypes i.e. receptors for gastrin-releasing peptide (GRPr) and neuromedin B (NMBr) differentially regulate itch scratching. However, to what degree spinal GRPr and NMBr regulate scratching evoked by intrathecally administered bombesin-related peptides is not known. The first aim of this study was to pharmacologically compare the dose-response curves for scratching induced by intrathecally administered bombesin-related peptides versus morphine, which is known to elicit itch in humans. The second aim was to determine if spinal GRPr and NMBr selectively or generally mediate scratching behavior. Mice received intrathecal injection of bombesin (0.01-0.3 nmol), GRP (0.01-0.3 nmol), NMB (0.1-1 nmol) or morphine (0.3-3 nmol) and were observed for one hour for scratching activity. Bombesin elicited most profound scratching over one hour followed by GRP and NMB, whereas morphine failed to evoke scratching response indicating the insensitivity of mouse models to intrathecal opioid-induced itch. Intrathecal pretreatment with GRPr antagonist RC-3095 (0.03-0.1 nmol) produced a parallel rightward shift in the dose response curve of GRP-induced scratching but not NMB-induced scratching. Similarly, PD168368 (1-3 nmol) only attenuated NMB but not GRP-induced scratching. Individual or co-administration of RC-3095 and PD168368 failed to alter bombesin-evoked scratching. A higher dose of RC-3095 (0.3 nmol) generally suppressed scratching induced by all three peptides but also compromised motor function in the rotarod test. Together, these data indicate that spinal GRPr and NMBr independently drive itch neurotransmission in mice and may not mediate bombesin-induced scratching. GRPr antagonists at functionally receptor-selective doses only block spinal GRP-elicited scratching but the suppression of scratching at higher doses is confounded by motor impairment.
Conflict of interest statement
Figures

Similar articles
-
The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats.J Pharmacol Exp Ther. 2011 Jun;337(3):822-9. doi: 10.1124/jpet.111.178970. Epub 2011 Mar 18. J Pharmacol Exp Ther. 2011. PMID: 21421741 Free PMC article.
-
Functional roles of neuromedin B and gastrin-releasing peptide in regulating itch and pain in the spinal cord of non-human primates.Biochem Pharmacol. 2022 Apr;198:114972. doi: 10.1016/j.bcp.2022.114972. Epub 2022 Feb 18. Biochem Pharmacol. 2022. PMID: 35189108 Free PMC article.
-
Spinal Functions of B-Type Natriuretic Peptide, Gastrin-Releasing Peptide, and Their Cognate Receptors for Regulating Itch in Mice.J Pharmacol Exp Ther. 2016 Mar;356(3):596-603. doi: 10.1124/jpet.115.229997. Epub 2015 Dec 15. J Pharmacol Exp Ther. 2016. PMID: 26669425 Free PMC article.
-
Gastrin-releasing peptide as a molecular _target for inflammatory diseases: an update.Inflamm Allergy Drug _targets. 2013 Jun;12(3):172-7. doi: 10.2174/1871528111312030003. Inflamm Allergy Drug _targets. 2013. PMID: 23621446 Review.
-
Gastrointestinal peptides and itch sensation.Curr Opin Endocrinol Diabetes Obes. 2015 Feb;22(1):29-33. doi: 10.1097/MED.0000000000000122. Curr Opin Endocrinol Diabetes Obes. 2015. PMID: 25485517 Review.
Cited by
-
Function of gastrin-releasing peptide receptors in ocular itch transmission in the mouse trigeminal sensory system.Front Mol Neurosci. 2023 Nov 30;16:1280024. doi: 10.3389/fnmol.2023.1280024. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38098939 Free PMC article.
-
Neuromedin B Induces Acute Itch in Mice via the Activation of Peripheral Sensory Neurons.Acta Derm Venereol. 2019 May 1;99(6):587-893. doi: 10.2340/00015555-3143. Acta Derm Venereol. 2019. PMID: 30734045 Free PMC article.
-
Altered expression of _target genes of spinal cord in different itch models compared with capsaicin assessed by RT-qPCR validation.Onco_target. 2017 Aug 10;8(43):74423-74433. doi: 10.18632/onco_target.20148. eCollection 2017 Sep 26. Onco_target. 2017. PMID: 29088797 Free PMC article.
-
Translational value of non-human primates in opioid research.Exp Neurol. 2021 Apr;338:113602. doi: 10.1016/j.expneurol.2021.113602. Epub 2021 Jan 14. Exp Neurol. 2021. PMID: 33453211 Free PMC article. Review.
-
Neuraxial opioid-induced itch and its pharmacological antagonism.Handb Exp Pharmacol. 2015;226:315-35. doi: 10.1007/978-3-662-44605-8_17. Handb Exp Pharmacol. 2015. PMID: 25861787 Free PMC article. Review.
References
-
- Weisshaar E, Dalgard F (2009) Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol 89: 339–350. - PubMed
-
- Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, et al. (2003) Itch: scratching more than the surface. Qjm 96: 7–26. - PubMed
-
- Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, et al. (2007) Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 87: 291–294. - PubMed
-
- Andoh T, Kuwazono T, Lee JB, Kuraishi Y (2011) Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides 32: 2098–2103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

