LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations
- PMID: 23836819
- PMCID: PMC3811755
- DOI: 10.1128/IAI.01288-12
LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations
Abstract
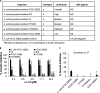
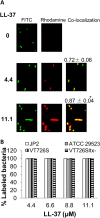
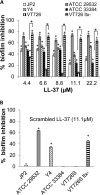
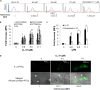
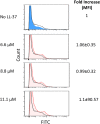
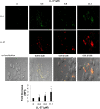
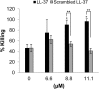
Host defense peptides are immediate responders of the innate immunity that express antimicrobial, immunoregulatory, and wound-healing activities. Neutrophils are a major source for oral host defense peptides, and phagocytosis by neutrophils is a major mechanism for bacterial clearance in the gingival tissue. Dysfunction of or reduction in the numbers of neutrophils or deficiency in the LL-37 host defense peptide was each previously linked with proliferation of oral Aggregatibacter actinomycetemcomitans which resulted in an aggressive periodontal disease. Surprisingly, A. actinomycetemcomitans shows resistance to high concentrations of LL-37. In this study, we demonstrated that submicrocidal concentrations of LL-37 inhibit biofilm formation by A. actinomycetemcomitans and act as opsonins and agglutinins that greatly enhance its clearance by neutrophils and macrophages. Improved uptake of A. actinomycetemcomitans by neutrophils was mediated by their opsonization with LL-37. Enhanced phagocytosis and killing of A. actinomycetemcomitans by murine macrophage-like RAW 264.7 cells were dependent on their preagglutination by LL-37. Although A. actinomycetemcomitans is resistant to the bactericidal effect of LL-37, our results offer a rationale for the epidemiological association between LL-37 deficiency and the expansion of oral A. actinomycetemcomitans and indicate a possible therapeutic use of cationic peptides for host defense.
Figures

Similar articles
-
Antibiofilm properties of cathelicidin LL-37: an in-depth review.World J Microbiol Biotechnol. 2023 Feb 14;39(4):99. doi: 10.1007/s11274-023-03545-z. World J Microbiol Biotechnol. 2023. PMID: 36781570 Review.
-
Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils.J Periodontal Res. 2007 Oct;42(5):410-9. doi: 10.1111/j.1600-0765.2006.00962.x. J Periodontal Res. 2007. PMID: 17760818
-
Synergistic effects of LFchimera and antibiotic against planktonic and biofilm form of Aggregatibacter actinomycetemcomitans.PLoS One. 2019 Jul 22;14(7):e0217205. doi: 10.1371/journal.pone.0217205. eCollection 2019. PLoS One. 2019. PMID: 31329599 Free PMC article.
-
Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: a cathelicidin found in human leukocytes and epithelium.Oral Microbiol Immunol. 2000 Aug;15(4):226-31. doi: 10.1034/j.1399-302x.2000.150403.x. Oral Microbiol Immunol. 2000. PMID: 11154407
-
High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
Cited by
-
Antimicrobial peptides: The miraculous biological molecules.J Indian Soc Periodontol. 2017 Nov-Dec;21(6):434-438. doi: 10.4103/jisp.jisp_325_16. J Indian Soc Periodontol. 2017. PMID: 29551860 Free PMC article. Review.
-
Gingival crevicular fluid and serum hCAP18/LL-37 levels in generalized aggressive periodontitis.Clin Oral Investig. 2017 Apr;21(3):763-769. doi: 10.1007/s00784-016-1834-z. Epub 2016 Apr 30. Clin Oral Investig. 2017. PMID: 27129587
-
Antibiofilm properties of cathelicidin LL-37: an in-depth review.World J Microbiol Biotechnol. 2023 Feb 14;39(4):99. doi: 10.1007/s11274-023-03545-z. World J Microbiol Biotechnol. 2023. PMID: 36781570 Review.
-
Alpha-melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide.Biomed Res Int. 2014;2014:874610. doi: 10.1155/2014/874610. Epub 2014 Jul 23. Biomed Res Int. 2014. PMID: 25140322 Free PMC article. Review.
-
Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination.Int J Mol Sci. 2024 Feb 24;25(5):2655. doi: 10.3390/ijms25052655. Int J Mol Sci. 2024. PMID: 38473900 Free PMC article. Review.
References
-
- Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 - PubMed
-
- Cole JN, Pence MA, von Kockritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, Nizet V. 2010. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. mBio 1(4):e00191-10.10.1128/mBio.00191-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

