Neuronal reprograming of protein homeostasis by calcium-dependent regulation of the heat shock response
- PMID: 24009518
- PMCID: PMC3757039
- DOI: 10.1371/journal.pgen.1003711
Neuronal reprograming of protein homeostasis by calcium-dependent regulation of the heat shock response
Abstract
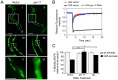
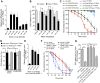
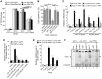
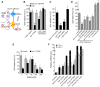
Protein quality control requires constant surveillance to prevent misfolding, aggregation, and loss of cellular function. There is increasing evidence in metazoans that communication between cells has an important role to ensure organismal health and to prevent stressed cells and tissues from compromising lifespan. Here, we show in C. elegans that a moderate increase in physiological cholinergic signaling at the neuromuscular junction (NMJ) induces the calcium (Ca(2+))-dependent activation of HSF-1 in post-synaptic muscle cells, resulting in suppression of protein misfolding. This protective effect on muscle cell protein homeostasis was identified in an unbiased genome-wide screening for modifiers of protein aggregation, and is triggered by downregulation of gei-11, a Myb-family factor and proposed regulator of the L-type acetylcholine receptor (AChR). This, in-turn, activates the voltage-gated Ca(2+) channel, EGL-19, and the sarcoplasmic reticulum ryanodine receptor in response to acetylcholine signaling. The release of calcium into the cytoplasm of muscle cells activates Ca(2+)-dependent kinases and induces HSF-1-dependent expression of cytoplasmic chaperones, which suppress misfolding of metastable proteins and stabilize the folding environment of muscle cells. This demonstrates that the heat shock response (HSR) can be activated in muscle cells by neuronal signaling across the NMJ to protect proteome health.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans.BMC Genomics. 2016 Aug 5;17:559. doi: 10.1186/s12864-016-2837-5. BMC Genomics. 2016. PMID: 27496166 Free PMC article.
-
The dystrophin-associated protein complex maintains muscle excitability by regulating Ca(2+)-dependent K(+) (BK) channel localization.J Biol Chem. 2011 Sep 23;286(38):33501-10. doi: 10.1074/jbc.M111.227678. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795674 Free PMC article.
-
Regulation of organismal proteostasis by transcellular chaperone signaling.Cell. 2013 Jun 6;153(6):1366-78. doi: 10.1016/j.cell.2013.05.015. Cell. 2013. PMID: 23746847 Free PMC article.
-
Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses.J Exp Biol. 2014 Jan 1;217(Pt 1):129-36. doi: 10.1242/jeb.091249. J Exp Biol. 2014. PMID: 24353212 Free PMC article. Review.
-
The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans.Int J Mol Sci. 2022 Nov 28;23(23):14907. doi: 10.3390/ijms232314907. Int J Mol Sci. 2022. PMID: 36499234 Free PMC article. Review.
Cited by
-
Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress.Int J Mol Sci. 2021 May 24;22(11):5551. doi: 10.3390/ijms22115551. Int J Mol Sci. 2021. PMID: 34074030 Free PMC article.
-
Cellular clearance of circulating transthyretin decreases cell-nonautonomous proteotoxicity in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7710-E7719. doi: 10.1073/pnas.1801117115. Epub 2018 Jul 30. Proc Natl Acad Sci U S A. 2018. PMID: 30061394 Free PMC article.
-
HSF-1: Guardian of the Proteome Through Integration of Longevity Signals to the Proteostatic Network.Front Aging. 2022 Jul 8;3:861686. doi: 10.3389/fragi.2022.861686. eCollection 2022. Front Aging. 2022. PMID: 35874276 Free PMC article. Review.
-
Neuronal aging: learning from C. elegans.J Mol Signal. 2013 Dec 10;8(1):14. doi: 10.1186/1750-2187-8-14. J Mol Signal. 2013. PMID: 24325838 Free PMC article.
-
Neuronal HLH-30/TFEB modulates peripheral mitochondrial fragmentation to improve thermoresistance in Caenorhabditis elegans.Aging Cell. 2023 Mar;22(3):e13741. doi: 10.1111/acel.13741. Epub 2022 Nov 23. Aging Cell. 2023. PMID: 36419219 Free PMC article.
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319: 916–919. - PubMed
-
- Morimoto RI (2012) The Heat Shock Response: Systems Biology of Proteotoxic Stress in Aging and Disease. In: Press CSH, editor. Cold Spring Harbor Symposia on Quantitative Biology: Metabolism and Disease. pp. 91–99. - PubMed
-
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311: 1471–1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

