Glial influence on the blood brain barrier
- PMID: 24123158
- PMCID: PMC4068281
- DOI: 10.1002/glia.22575
Glial influence on the blood brain barrier
Abstract
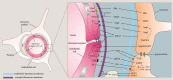
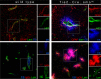
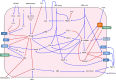
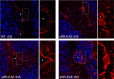
The Blood Brain Barrier (BBB) is a specialized vascular structure tightly regulating central nervous system (CNS) homeostasis. Endothelial cells are the central component of the BBB and control of their barrier phenotype resides on astrocytes and pericytes. Interactions between these cells and the endothelium promote and maintain many of the physiological and metabolic characteristics that are unique to the BBB. In this review we describe recent findings related to the involvement of astroglial cells, including radial glial cells, in the induction of barrier properties during embryogenesis and adulthood. In addition, we describe changes that occur in astrocytes and endothelial cells during injury and inflammation with a particular emphasis on alterations of the BBB phenotype.
Keywords: BBB; astrocyte; astrogliosis; glial; multiple sclerosis.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

Similar articles
-
Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes.Diabetes Metab J. 2022 Mar;46(2):222-238. doi: 10.4093/dmj.2021.0146. Epub 2022 Mar 18. Diabetes Metab J. 2022. PMID: 35299293 Free PMC article. Review.
-
Glial-cell-mediated re-induction of the blood-brain barrier phenotype in brain capillary endothelial cells: a differential gel electrophoresis study.Proteomics. 2013 Apr;13(7):1185-99. doi: 10.1002/pmic.201200166. Proteomics. 2013. PMID: 23436736
-
Morphofunctional aspects of the blood-brain barrier.Curr Drug Metab. 2012 Jan;13(1):50-60. doi: 10.2174/138920012798356970. Curr Drug Metab. 2012. PMID: 22292807 Review.
-
Blood-brain barrier interfaces and brain tumors.Arch Pharm Res. 2006 Apr;29(4):265-75. doi: 10.1007/BF02968569. Arch Pharm Res. 2006. PMID: 16681030 Review.
-
Connexin Channels at the Glio-Vascular Interface: Gatekeepers of the Brain.Neurochem Res. 2017 Sep;42(9):2519-2536. doi: 10.1007/s11064-017-2313-x. Epub 2017 Jun 20. Neurochem Res. 2017. PMID: 28634726 Review.
Cited by
-
Transgenic animal models to explore and modulate the blood brain and blood retinal barriers of the CNS.Fluids Barriers CNS. 2022 Nov 1;19(1):86. doi: 10.1186/s12987-022-00386-0. Fluids Barriers CNS. 2022. PMID: 36320068 Free PMC article. Review.
-
Circulating GFAP and Iba-1 levels are associated with pathophysiological sequelae in the thalamus in a pig model of mild TBI.Sci Rep. 2020 Aug 7;10(1):13369. doi: 10.1038/s41598-020-70266-w. Sci Rep. 2020. PMID: 32770054 Free PMC article.
-
Regulation of human glia by multiple sclerosis disease modifying therapies.Semin Immunopathol. 2015 Nov;37(6):639-49. doi: 10.1007/s00281-015-0514-4. Epub 2015 Aug 11. Semin Immunopathol. 2015. PMID: 26259734 Review.
-
Amyloid precursor protein induces reactive astrogliosis.bioRxiv [Preprint]. 2023 Dec 18:2023.12.18.571817. doi: 10.1101/2023.12.18.571817. bioRxiv. 2023. Update in: Acta Physiol (Oxf). 2024 Jun;240(6):e14142. doi: 10.1111/apha.14142 PMID: 38187544 Free PMC article. Updated. Preprint.
-
Transient Opening of the Blood-Brain Barrier by Vasoactive Peptides to Increase CNS Drug Delivery: Reality Versus Wishful Thinking?Curr Neuropharmacol. 2022;20(7):1383-1399. doi: 10.2174/1570159X20999220131163504. Curr Neuropharmacol. 2022. PMID: 35100958 Free PMC article. Review.
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Adams RH. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin Cell Dev Biol. 2002;13:55–60. - PubMed
-
- Adner M, Jansen I, Edvinsson L. Endothelin-A receptors mediate contraction in human cerebral, meningeal and temporal arteries. J Auton Nerv Syst. 1994;49(Suppl):S117–S121. - PubMed
-
- Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, Hulit J, Sakamaki T, Fu M, Ben-Ze'ev A, Bromberg JF, Lamberti C, Verma U, Gaynor RB, Byers SW, Pestell RG. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

