The mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M) and glucose homeostasis: has it been overlooked?
- PMID: 24177027
- PMCID: PMC3943549
- DOI: 10.1016/j.bbagen.2013.10.033
The mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M) and glucose homeostasis: has it been overlooked?
Abstract
Background: Plasma glucose levels are tightly regulated within a narrow physiologic range. Insulin-mediated glucose uptake by tissues must be balanced by the appearance of glucose from nutritional sources, glycogen stores, or gluconeogenesis. In this regard, a common pathway regulating both glucose clearance and appearance has not been described. The metabolism of glucose to produce ATP is generally considered to be the primary stimulus for insulin release from beta-cells. Similarly, gluconeogenesis from phosphoenolpyruvate (PEP) is believed to be the primarily pathway via the cytosolic isoform of phosphoenolpyruvate carboxykinase (PEPCK-C). These models cannot adequately explain the regulation of insulin secretion or gluconeogenesis.
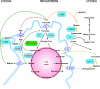
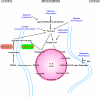
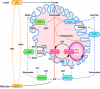
Scope of review: A metabolic sensing pathway involving mitochondrial GTP (mtGTP) and PEP synthesis by the mitochondrial isoform of PEPCK (PEPCK-M) is associated with glucose-stimulated insulin secretion from pancreatic beta-cells. Here we examine whether there is evidence for a similar mtGTP-dependent pathway involved in gluconeogenesis. In both islets and the liver, mtGTP is produced at the substrate level by the enzyme succinyl CoA synthetase (SCS-GTP) with a rate proportional to the TCA cycle. In the beta-cell PEPCK-M then hydrolyzes mtGTP in the production of PEP that, unlike mtGTP, can escape the mitochondria to generate a signal for insulin release. Similarly, PEPCK-M and mtGTP might also provide a significant source of PEP in gluconeogenic tissues for the production of glucose. This review will focus on the possibility that PEPCK-M, as a sensor for TCA cycle flux, is a key mechanism to regulate both insulin secretion and gluconeogenesis suggesting conservation of this biochemical mechanism in regulating multiple aspects of glucose homeostasis. Moreover, we propose that this mechanism may be important for regulating insulin secretion and gluconeogenesis compared to canonical nutrient sensing pathways.
Major conclusions: PEPCK-M, initially believed to be absent in islets, carries a substantial metabolic flux in beta-cells. This flux is intimately involved with the coupling of glucose-stimulated insulin secretion. PEPCK-M activity may have been similarly underestimated in glucose producing tissues and could potentially be an unappreciated but important source of gluconeogenesis.
General significance: The generation of PEP via PEPCK-M may occur via a metabolic sensing pathway important for regulating both insulin secretion and gluconeogenesis. This article is part of a Special Issue entitled Frontiers of Mitochondrial Research.
Keywords: Anaplerosis; Gluconeogenesis; Insulin secretion; Mitochondrial GTP; PEPCK-M; Succinyl coenzyme A synthetase.
© 2013.
Figures




Similar articles
-
Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion.J Biol Chem. 2009 Sep 25;284(39):26578-90. doi: 10.1074/jbc.M109.011775. Epub 2009 Jul 27. J Biol Chem. 2009. PMID: 19635791 Free PMC article.
-
A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis.J Biol Chem. 2014 Mar 14;289(11):7257-63. doi: 10.1074/jbc.C113.544759. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497630 Free PMC article.
-
Mitochondrial GTP Links Nutrient Sensing to β Cell Health, Mitochondrial Morphology, and Insulin Secretion Independent of OxPhos.Cell Rep. 2019 Jul 16;28(3):759-772.e10. doi: 10.1016/j.celrep.2019.06.058. Cell Rep. 2019. PMID: 31315053 Free PMC article.
-
PEPCK and glucose metabolism homeostasis in arthropods.Insect Biochem Mol Biol. 2023 Sep;160:103986. doi: 10.1016/j.ibmb.2023.103986. Epub 2023 Jul 16. Insect Biochem Mol Biol. 2023. PMID: 37454751 Review.
-
Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression.Annu Rev Biochem. 1997;66:581-611. doi: 10.1146/annurev.biochem.66.1.581. Annu Rev Biochem. 1997. PMID: 9242918 Review.
Cited by
-
Mitochondrial Phosphoenolpyruvate Carboxykinase Regulates Osteogenic Differentiation by Modulating AMPK/ULK1-Dependent Autophagy.Stem Cells. 2019 Dec;37(12):1542-1555. doi: 10.1002/stem.3091. Epub 2019 Oct 14. Stem Cells. 2019. PMID: 31574189 Free PMC article.
-
Glucose Regulation of β-Cell KATP Channels: It Is Time for a New Model!Diabetes. 2024 Jun 1;73(6):856-863. doi: 10.2337/dbi23-0032. Diabetes. 2024. PMID: 38768366 Review.
-
Is serum biotinidase enzyme activity a potential marker of perturbed glucose and lipid metabolism?JIMD Rep. 2020 Oct 6;57(1):58-66. doi: 10.1002/jmd2.12168. eCollection 2021 Jan. JIMD Rep. 2020. PMID: 33473341 Free PMC article.
-
Simulation of the crosstalk between glucose and acetaminophen metabolism in a liver zonation model.Front Pharmacol. 2022 Sep 23;13:995597. doi: 10.3389/fphar.2022.995597. eCollection 2022. Front Pharmacol. 2022. PMID: 36210818 Free PMC article.
-
Heat Stress Induces Alterations in Gene Expression of Actin Cytoskeleton and Filament of Cellular Components Causing Gut Disruption in Growing-Finishing Pigs.Animals (Basel). 2024 Aug 26;14(17):2476. doi: 10.3390/ani14172476. Animals (Basel). 2024. PMID: 39272260 Free PMC article.
References
-
- Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol. 1999;276(1 Pt 1):E78–84. - PubMed
-
- Meyer C, et al. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol. 1998;275(6 Pt 2):F915–21. - PubMed
-
- Jones JG, et al. Hepatic anaplerotic outflow fluxes are redirected from gluconeogenesis to lactate synthesis in patients with Type 1a glycogen storage disease. Metabolic engineering. 2009;11(3):155–62. - PubMed
-
- Magnusson I, et al. Noninvasive tracing of Krebs cycle metabolism in liver. The Journal of biological chemistry. 1991;266(11):6975–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

