CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo
- PMID: 24179152
- PMCID: PMC3971079
- DOI: 10.3324/haematol.2013.093187
CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo
Abstract
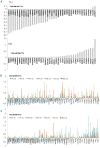
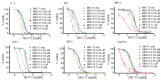
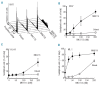
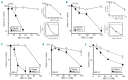
Novel combinations _targeting new molecular vulnerabilities are needed to improve the outcome of patients with acute myeloid leukemia. We recently identified WEE1 kinase as a novel _target in leukemias. To identify genes that are synthetically lethal with WEE1 inhibition, we performed a short interfering RNA screen directed against cell cycle and DNA repair genes during concurrent treatment with the WEE1 inhibitor MK1775. CHK1 and ATR, genes encoding two replication checkpoint kinases, were among the genes whose silencing enhanced the effects of WEE1 inhibition most, whereas CDK2 short interfering RNA antagonized MK1775 effects. Building on this observation, we examined the impact of combining MK1775 with selective small molecule inhibitors of CHK1, ATR and cyclin-dependent kinases. The CHK1 inhibitor MK8776 sensitized acute myeloid leukemia cell lines and primary leukemia specimens to MK1775 ex vivo, whereas smaller effects were observed with the MK1775/MK8776 combination in normal myeloid progenitors. The ATR inhibitor VE-821 likewise enhanced the antiproliferative effects of MK1775, whereas the cyclin-dependent kinase inhibitor roscovitine antagonized MK1775. Further studies showed that MK8776 enhanced MK1775-mediated activation of the ATR/CHK1 pathway in acute leukemia cell lines and ex vivo. These results indicate that combined cell cycle checkpoint interference with MK1775/MK8776 warrants further investigation as a potential treatment for acute myeloid leukemia.
Figures


Similar articles
-
Chk1 inhibition and Wee1 inhibition combine synergistically to impede cellular proliferation.Cancer Biol Ther. 2011 Nov 1;12(9):788-96. doi: 10.4161/cbt.12.9.17673. Epub 2011 Nov 1. Cancer Biol Ther. 2011. PMID: 21892012
-
Combined inhibition of Chk1 and Wee1: in vitro synergistic effect translates to tumor growth inhibition in vivo.Cell Cycle. 2012 Jul 1;11(13):2507-17. doi: 10.4161/cc.20899. Epub 2012 Jul 1. Cell Cycle. 2012. PMID: 22713237
-
Pharmacological _targeting the ATR-CHK1-WEE1 axis involves balancing cell growth stimulation and apoptosis.Onco_target. 2014 Nov 15;5(21):10546-57. doi: 10.18632/onco_target.2508. Onco_target. 2014. PMID: 25301733 Free PMC article.
-
Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition.Cancer Treat Rev. 2018 Dec;71:1-7. doi: 10.1016/j.ctrv.2018.09.003. Epub 2018 Sep 11. Cancer Treat Rev. 2018. PMID: 30269007 Free PMC article. Review.
-
Wee1 kinase as a _target for cancer therapy.Cell Cycle. 2013 Oct 1;12(19):3159-64. doi: 10.4161/cc.26062. Epub 2013 Aug 26. Cell Cycle. 2013. PMID: 24013427 Free PMC article. Review.
Cited by
-
Chemogenetic profiling identifies RAD17 as synthetically lethal with checkpoint kinase inhibition.Onco_target. 2015 Nov 3;6(34):35755-69. doi: 10.18632/onco_target.5928. Onco_target. 2015. PMID: 26437225 Free PMC article.
-
Synergistic anti-leukemic interactions between panobinostat and MK-1775 in acute myeloid leukemia ex vivo.Cancer Biol Ther. 2015;16(12):1784-93. doi: 10.1080/15384047.2015.1095406. Cancer Biol Ther. 2015. PMID: 26529495 Free PMC article.
-
Characterizing functional DNA damage and response caused by the combination of CHK1 and WEE1 inhibitors in ovarian and breast cancer models.BJC Rep. 2024 Apr 3;2(1):27. doi: 10.1038/s44276-024-00048-8. BJC Rep. 2024. PMID: 39516567 Free PMC article.
-
Randomized phase II trial of cytosine arabinoside with and without the CHK1 inhibitor MK-8776 in relapsed and refractory acute myeloid leukemia.Leuk Res. 2017 Oct;61:108-116. doi: 10.1016/j.leukres.2017.09.005. Epub 2017 Sep 20. Leuk Res. 2017. PMID: 28957699 Free PMC article. Clinical Trial.
-
Co-delivery of Aurora-A inhibitor XY-4 and Bcl-xl siRNA enhances antitumor efficacy for melanoma therapy.Int J Nanomedicine. 2018 Mar 9;13:1443-1456. doi: 10.2147/IJN.S147759. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29563798 Free PMC article.
References
-
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487–94 - PubMed
-
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–9 - PubMed
-
- Cavelier C, Didier C, Prade N, Mansat-De Mas V, Manenti S, Recher C, et al. Constitutive activation of the DNA damage signaling pathway in acute myeloid leukemia with complex karyotype: potential importance for checkpoint _targeting therapy. Cancer Res. 2009;69(22):8652–61 - PubMed
-
- Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23(1):203–6 - PubMed
-
- Seifert H, Mohr B, Thiede C, Oelschlagel U, Schakel U, Illmer T, et al. The prognostic impact of 17p (p53) deletion in 2272 adults with acute myeloid leukemia. Leukemia. 2009;23(4):656–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

