The cyclic AMP phosphodiesterase RegA critically regulates encystation in social and pathogenic amoebas
- PMID: 24184654
- PMCID: PMC3906536
- DOI: 10.1016/j.cellsig.2013.10.008
The cyclic AMP phosphodiesterase RegA critically regulates encystation in social and pathogenic amoebas
Abstract
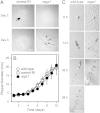
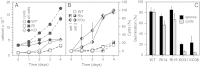
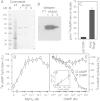
Amoebas survive environmental stress by differentiating into encapsulated cysts. As cysts, pathogenic amoebas resist antibiotics, which particularly counteracts treatment of vision-destroying Acanthamoeba keratitis. Limited genetic tractability of amoeba pathogens has left their encystation mechanisms unexplored. The social amoeba Dictyostelium discoideum forms spores in multicellular fruiting bodies to survive starvation, while other dictyostelids, such as Polysphondylium pallidum can additionally encyst as single cells. Sporulation is induced by cAMP acting on PKA, with the cAMP phosphodiesterase RegA critically regulating cAMP levels. We show here that RegA is deeply conserved in social and pathogenic amoebas and that deletion of the RegA gene in P. pallidum causes precocious encystation and prevents cyst germination. We heterologously expressed and characterized Acanthamoeba RegA and performed a compound screen to identify RegA inhibitors. Two effective inhibitors increased cAMP levels and triggered Acanthamoeba encystation. Our results show that RegA critically regulates Amoebozoan encystation and that components of the cAMP signalling pathway could be effective _targets for therapeutic intervention with encystation.
Keywords: 3′5′-adenosine monophosphate; ACG; Acanthamoeba castellani; Acanthamoeba keratitis; Acas; AcrA; Ddis; Dictyostelium discoideum; Encystation; KO; MRSA; PDE; PKA; Polysphondylium pallidum; Ppal; RI; Sensor histidine kinase; Stress signalling; adenylate cyclase G; adenylate cyclase R; cAMP; cAMP dependent protein kinase; cAMP-phosphodiesterase; knock-out; methicillin resistant Streptococcus aureus; phosphodiesterase; random integrant.
© 2013. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The social amoeba Polysphondylium pallidum loses encystation and sporulation, but can still erect fruiting bodies in the absence of cellulose.Protist. 2014 Sep;165(5):569-79. doi: 10.1016/j.protis.2014.07.003. Epub 2014 Jul 14. Protist. 2014. PMID: 25113829 Free PMC article.
-
Loss of cAMP-specific phosphodiesterase rescues spore development in G protein mutant in dictyostelium.Cell Signal. 2014 Feb;26(2):409-18. doi: 10.1016/j.cellsig.2013.10.003. Cell Signal. 2014. PMID: 24511612 Free PMC article.
-
Mitogen-activated protein kinase regulation of the phosphodiesterase RegA in early Dictyostelium development.Microbiology (Reading). 2020 Feb;166(2):129-140. doi: 10.1099/mic.0.000868. Microbiology (Reading). 2020. PMID: 31730032 Free PMC article.
-
Encystation: the most prevalent and underinvestigated differentiation pathway of eukaryotes.Microbiology (Reading). 2018 May;164(5):727-739. doi: 10.1099/mic.0.000653. Epub 2018 Apr 5. Microbiology (Reading). 2018. PMID: 29620506 Review.
-
The Evolution of Aggregative Multicellularity and Cell-Cell Communication in the Dictyostelia.J Mol Biol. 2015 Nov 20;427(23):3722-33. doi: 10.1016/j.jmb.2015.08.008. Epub 2015 Aug 15. J Mol Biol. 2015. PMID: 26284972 Free PMC article. Review.
Cited by
-
The Origin of Animal Multicellularity and Cell Differentiation.Dev Cell. 2017 Oct 23;43(2):124-140. doi: 10.1016/j.devcel.2017.09.016. Dev Cell. 2017. PMID: 29065305 Free PMC article. Review.
-
Variables Affecting the Recovery of Acanthamoeba Trophozoites.Pathogens. 2021 Feb 18;10(2):221. doi: 10.3390/pathogens10020221. Pathogens. 2021. PMID: 33670669 Free PMC article.
-
Evolution of multicellularity in Dictyostelia.Int J Dev Biol. 2019;63(8-9-10):359-369. doi: 10.1387/ijdb.190108ps. Int J Dev Biol. 2019. PMID: 31840775 Free PMC article. Review.
-
Emerging roles for diguanylate cyclase during the evolution of soma in dictyostelia.BMC Ecol Evol. 2023 Oct 6;23(1):60. doi: 10.1186/s12862-023-02169-z. BMC Ecol Evol. 2023. PMID: 37803310 Free PMC article.
-
The social amoeba Polysphondylium pallidum loses encystation and sporulation, but can still erect fruiting bodies in the absence of cellulose.Protist. 2014 Sep;165(5):569-79. doi: 10.1016/j.protis.2014.07.003. Epub 2014 Jul 14. Protist. 2014. PMID: 25113829 Free PMC article.
References
-
- Turner N.A., Biagini G.A., Lloyd D. FEMS Microbiol. Lett. 1997;157:149–153.
-
- Kumar R., Lloyd D. Clin. Infect. Dis. 2002;35:434–441. - PubMed
-
- Lloyd D., Turner N.A., Khunkitti W., Hann A.C., Furr J.R., Russell A.D. J. Eukaryot. Microbiol. 2001;48:11–16. - PubMed
-
- Visvesvara G.S. Curr. Opin. Infect. Dis. 2010;23:590–594. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

