A fluorescent curcumin-based Zn(II)-complex reactivates mutant (R175H and R273H) p53 in cancer cells
- PMID: 24220325
- PMCID: PMC3851540
- DOI: 10.1186/1756-9966-32-72
A fluorescent curcumin-based Zn(II)-complex reactivates mutant (R175H and R273H) p53 in cancer cells
Abstract
Background: Mutations of the p53 oncosuppressor gene are amongst the most frequent aberration seen in human cancer. Some mutant (mt) p53 proteins are prone to loss of Zn(II) ion that is bound to the wild-type (wt) core, promoting protein aggregation and therefore unfolding. Misfolded p53 protein conformation impairs wtp53-DNA binding and transactivation activities, favouring tumor growth and resistance to antitumor therapies. Screening studies, devoted to identify small molecules that reactivate mtp53, represent therefore an attractive anti-cancer therapeutic strategy. Here we tested a novel fluorescent curcumin-based Zn(II)-complex (Zn-curc) to evaluate its effect on mtp53 reactivation in cancer cells.
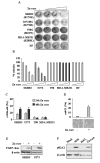
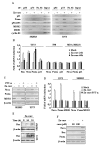
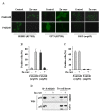
Methods: P53 protein conformation was examined after Zn-curc treatment by immunoprecipitation and immunofluorescence assays, using conformation-specific antibodies. The mtp53 reactivation was evaluated by chromatin-immunoprecipitation (ChIP) and semi-quantitative RT-PCR analyses of wild-type p53 _target genes. The intratumoral Zn-curc localization was evaluated by immunofluorescence analysis of glioblastoma tissues of an ortothopic mice model.
Results: The Zn-curc complex induced conformational change in p53-R175H and -R273H mutant proteins, two of the most common p53 mutations. Zn-curc treatment restored wtp53-DNA binding and transactivation functions and induced apoptotic cell death. In vivo studies showed that the Zn-curc complex reached glioblastoma tissues of an ortothopic mice model, highlighting its ability to crossed the blood-tumor barrier.
Conclusions: Our results demonstrate that Zn-curc complex may reactivate specific mtp53 proteins and that may cross the blood-tumor barrier, becoming a promising compound for the development of drugs to halt tumor growth.
Figures

Similar articles
-
Zn(II)-curc _targets p53 in thyroid cancer cells.Int J Oncol. 2015 Oct;47(4):1241-8. doi: 10.3892/ijo.2015.3125. Epub 2015 Aug 13. Int J Oncol. 2015. PMID: 26314369 Free PMC article.
-
Restoring p53 active conformation by zinc increases the response of mutant p53 tumor cells to anticancer drugs.Cell Cycle. 2011 May 15;10(10):1679-89. doi: 10.4161/cc.10.10.15642. Epub 2011 May 15. Cell Cycle. 2011. PMID: 21508668
-
Degradation of mutant p53H175 protein by Zn(II) through autophagy.Cell Death Dis. 2014 May 29;5(5):e1271. doi: 10.1038/cddis.2014.217. Cell Death Dis. 2014. PMID: 24874727 Free PMC article.
-
Role of Reactivating Mutant p53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer.Onco _targets Ther. 2022 Jan 8;15:23-30. doi: 10.2147/OTT.S342292. eCollection 2022. Onco _targets Ther. 2022. PMID: 35035222 Free PMC article. Review.
-
Dual Modulators of p53 and Cyclin D in ER Alpha Signaling by Albumin Nanovectors Bearing Zinc Chaperones for ER-positive Breast Cancer Therapy.Mini Rev Med Chem. 2021;21(7):792-802. doi: 10.2174/1389557520999201124212347. Mini Rev Med Chem. 2021. PMID: 33238842 Review.
Cited by
-
Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer.Biomed Res Int. 2015;2015:878134. doi: 10.1155/2015/878134. Epub 2015 Mar 23. Biomed Res Int. 2015. PMID: 25879038 Free PMC article.
-
Metal-Based Anticancer Complexes and p53: How Much Do We Know?Cancers (Basel). 2023 May 19;15(10):2834. doi: 10.3390/cancers15102834. Cancers (Basel). 2023. PMID: 37345171 Free PMC article. Review.
-
Aggregation and Prion-Like Properties of Misfolded Tumor Suppressors: Is Cancer a Prion Disease?Cold Spring Harb Perspect Biol. 2016 Oct 3;8(10):a023614. doi: 10.1101/cshperspect.a023614. Cold Spring Harb Perspect Biol. 2016. PMID: 27549118 Free PMC article. Review.
-
A role for bioinorganic chemistry in the reactivation of mutant p53 in cancer.J Biol Inorg Chem. 2022 Aug;27(4-5):393-403. doi: 10.1007/s00775-022-01939-2. Epub 2022 Apr 30. J Biol Inorg Chem. 2022. PMID: 35488931 Review.
-
Small molecule restoration of wildtype structure and function of mutant p53 using a novel zinc-metallochaperone based mechanism.Onco_target. 2014 Oct 15;5(19):8879-92. doi: 10.18632/onco_target.2432. Onco_target. 2014. PMID: 25294809 Free PMC article.
References
-
- Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53 and cancer-associated mutants. Adv Cancer Res. 2007;97:1–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

