K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions
- PMID: 24256730
- PMCID: PMC4274051
- DOI: 10.1038/nature12796
K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions
Abstract
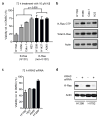
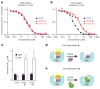
Somatic mutations in the small GTPase K-Ras are the most common activating lesions found in human cancer, and are generally associated with poor response to standard therapies. Efforts to _target this oncogene directly have faced difficulties owing to its picomolar affinity for GTP/GDP and the absence of known allosteric regulatory sites. Oncogenic mutations result in functional activation of Ras family proteins by impairing GTP hydrolysis. With diminished regulation by GTPase activity, the nucleotide state of Ras becomes more dependent on relative nucleotide affinity and concentration. This gives GTP an advantage over GDP and increases the proportion of active GTP-bound Ras. Here we report the development of small molecules that irreversibly bind to a common oncogenic mutant, K-Ras(G12C). These compounds rely on the mutant cysteine for binding and therefore do not affect the wild-type protein. Crystallographic studies reveal the formation of a new pocket that is not apparent in previous structures of Ras, beneath the effector binding switch-II region. Binding of these inhibitors to K-Ras(G12C) disrupts both switch-I and switch-II, subverting the native nucleotide preference to favour GDP over GTP and impairing binding to Raf. Our data provide structure-based validation of a new allosteric regulatory site on Ras that is _targetable in a mutant-specific manner.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures


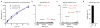
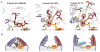



Comment in
-
Drug discovery: Pocket of opportunity.Nature. 2013 Nov 28;503(7477):475-6. doi: 10.1038/nature12835. Epub 2013 Nov 20. Nature. 2013. PMID: 24256732 No abstract available.
-
Oncogenes: direct hit on mutant RAS.Nat Rev Cancer. 2014 Jan;14(1):8-9. doi: 10.1038/nrc3650. Epub 2013 Dec 5. Nat Rev Cancer. 2014. PMID: 24304874 No abstract available.
Similar articles
-
Drug discovery: Pocket of opportunity.Nature. 2013 Nov 28;503(7477):475-6. doi: 10.1038/nature12835. Epub 2013 Nov 20. Nature. 2013. PMID: 24256732 No abstract available.
-
Oncogenes: direct hit on mutant RAS.Nat Rev Cancer. 2014 Jan;14(1):8-9. doi: 10.1038/nrc3650. Epub 2013 Dec 5. Nat Rev Cancer. 2014. PMID: 24304874 No abstract available.
-
In situ selectivity profiling and crystal structure of SML-8-73-1, an active site inhibitor of oncogenic K-Ras G12C.Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8895-900. doi: 10.1073/pnas.1404639111. Epub 2014 Jun 2. Proc Natl Acad Sci U S A. 2014. PMID: 24889603 Free PMC article.
-
Mutant-Specific _targeting of Ras G12C Activity by Covalently Reacting Small Molecules.Cell Chem Biol. 2019 Oct 17;26(10):1338-1348. doi: 10.1016/j.chembiol.2019.07.005. Epub 2019 Aug 1. Cell Chem Biol. 2019. PMID: 31378709 Review.
-
Three-dimensional structure of p21 in the active conformation and analysis of an oncogenic mutant.Environ Health Perspect. 1991 Jun;93:11-5. doi: 10.1289/ehp.919311. Environ Health Perspect. 1991. PMID: 1773783 Free PMC article. Review.
Cited by
-
Delineating cysteine-reactive compound modulation of cellular proteostasis processes.Nat Chem Biol. 2024 Oct 24. doi: 10.1038/s41589-024-01760-9. Online ahead of print. Nat Chem Biol. 2024. PMID: 39448844
-
Discovering and _targeting Dynamic Drugging Pockets of Oncogenic Proteins: The Role of Magnesium in Conformational Changes of the G12D Mutated Kirsten Rat Sarcoma-Guanosine Diphosphate Complex.Int J Mol Sci. 2022 Nov 10;23(22):13865. doi: 10.3390/ijms232213865. Int J Mol Sci. 2022. PMID: 36430338 Free PMC article.
-
Combined rational design and a high throughput screening platform for identifying chemical inhibitors of a Ras-activating enzyme.J Biol Chem. 2015 May 15;290(20):12879-98. doi: 10.1074/jbc.M114.634493. Epub 2015 Mar 30. J Biol Chem. 2015. PMID: 25825487 Free PMC article.
-
Determining KRAS4B-_targeting Compound Specificity by Top-Down Mass Spectrometry.Methods Mol Biol. 2024;2823:291-310. doi: 10.1007/978-1-0716-3922-1_18. Methods Mol Biol. 2024. PMID: 39052227
-
Sotorasib for Lung Cancers with KRAS p.G12C Mutation.N Engl J Med. 2021 Jun 24;384(25):2371-2381. doi: 10.1056/NEJMoa2103695. Epub 2021 Jun 4. N Engl J Med. 2021. PMID: 34096690 Free PMC article. Clinical Trial.
References
-
- Slebos RJC, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–565. - PubMed
-
- Lièvre A, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. - PubMed
-
- John J, et al. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry. 1990;29:6058–6065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

