A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway
- PMID: 24267888
- PMCID: PMC3873608
- DOI: 10.1016/j.cell.2013.10.022
A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway
Abstract
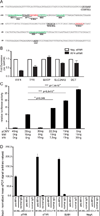
Sequence polymorphisms linked to human diseases and phenotypes in genome-wide association studies often affect noncoding regions. A SNP within an intron of the gene encoding Interferon Regulatory Factor 4 (IRF4), a transcription factor with no known role in melanocyte biology, is strongly associated with sensitivity of skin to sun exposure, freckles, blue eyes, and brown hair color. Here, we demonstrate that this SNP lies within an enhancer of IRF4 transcription in melanocytes. The allele associated with this pigmentation phenotype impairs binding of the TFAP2A transcription factor that, together with the melanocyte master regulator MITF, regulates activity of the enhancer. Assays in zebrafish and mice reveal that IRF4 cooperates with MITF to activate expression of Tyrosinase (TYR), an essential enzyme in melanin synthesis. Our findings provide a clear example of a noncoding polymorphism that affects a phenotype by modulating a developmental gene regulatory network.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Comment in
-
SNPing away at human skin color.Pigment Cell Melanoma Res. 2014 May;27(3):322-3. doi: 10.1111/pcmr.12229. Epub 2014 Mar 3. Pigment Cell Melanoma Res. 2014. PMID: 24517848 No abstract available.
Similar articles
-
TFAP2 paralogs regulate melanocyte differentiation in parallel with MITF.PLoS Genet. 2017 Mar 1;13(3):e1006636. doi: 10.1371/journal.pgen.1006636. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28249010 Free PMC article.
-
Allele-specific transcriptional regulation of IRF4 in melanocytes is mediated by chromatin looping of the intronic rs12203592 enhancer to the IRF4 promoter.Hum Mol Genet. 2015 May 1;24(9):2649-61. doi: 10.1093/hmg/ddv029. Epub 2015 Jan 27. Hum Mol Genet. 2015. PMID: 25631878
-
Genetic variation in IRF4 expression modulates growth characteristics, tyrosinase expression and interferon-gamma response in melanocytic cells.Pigment Cell Melanoma Res. 2018 Jan;31(1):51-63. doi: 10.1111/pcmr.12620. Epub 2017 Oct 23. Pigment Cell Melanoma Res. 2018. PMID: 28755520
-
Molecular genetics of human pigmentation diversity.Hum Mol Genet. 2009 Apr 15;18(R1):R9-17. doi: 10.1093/hmg/ddp003. Hum Mol Genet. 2009. PMID: 19297406 Review.
-
Beyond MITF: Multiple transcription factors directly regulate the cellular phenotype in melanocytes and melanoma.Pigment Cell Melanoma Res. 2017 Sep;30(5):454-466. doi: 10.1111/pcmr.12611. Pigment Cell Melanoma Res. 2017. PMID: 28649789 Free PMC article. Review.
Cited by
-
Genomic analysis reveals distinct mechanisms and functional classes of SOX10-regulated genes in melanocytes.Hum Mol Genet. 2015 Oct 1;24(19):5433-50. doi: 10.1093/hmg/ddv267. Epub 2015 Jul 23. Hum Mol Genet. 2015. PMID: 26206884 Free PMC article.
-
Association of Interferon Regulatory Factor-4 Polymorphism rs12203592 With Divergent Melanoma Pathways.J Natl Cancer Inst. 2016 Feb 8;108(7):djw004. doi: 10.1093/jnci/djw004. Print 2016 Jul. J Natl Cancer Inst. 2016. PMID: 26857527 Free PMC article.
-
Dissecting dynamics and differences of selective pressures in the evolution of human pigmentation.Biol Open. 2021 Feb 9;10(2):bio056523. doi: 10.1242/bio.056523. Biol Open. 2021. PMID: 33495209 Free PMC article.
-
Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases.Pharmaceutics. 2022 Oct 27;14(11):2308. doi: 10.3390/pharmaceutics14112308. Pharmaceutics. 2022. PMID: 36365128 Free PMC article. Review.
-
Goat Genomic Resources: The Search for Genes Associated with Its Economic Traits.Int J Genomics. 2020 Aug 18;2020:5940205. doi: 10.1155/2020/5940205. eCollection 2020. Int J Genomics. 2020. PMID: 32904540 Free PMC article. Review.
References
-
- Adachi S, Morii E, Kim D, Ogihara H, Jippo T, Ito A, Lee YM, Kitamura Y. Involvement of mi-transcription factor in expression of alpha-melanocyte-stimulating hormone receptor in cultured mast cells of mice. Journal of immunology. 2000;164:855–860. - PubMed
-
- Aoki H, Moro O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R) Life Sci. 2002;71:2171–2179. - PubMed
-
- Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, Bouwes Bavinck JN. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

