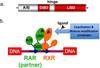
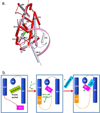
Retinoic acid actions through mammalian nuclear receptors
- PMID: 24308533
- PMCID: PMC3931982
- DOI: 10.1021/cr400161b
Retinoic acid actions through mammalian nuclear receptors
Figures


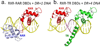
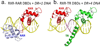




Similar articles
-
Docking simulations suggest that all-trans retinoic acid could bind to retinoid X receptors.J Comput Aided Mol Des. 2015 Oct;29(10):975-88. doi: 10.1007/s10822-015-9869-9. Epub 2015 Sep 18. J Comput Aided Mol Des. 2015. PMID: 26384496
-
Architecture of DNA Bound RAR heterodimers.Subcell Biochem. 2014;70:21-36. doi: 10.1007/978-94-017-9050-5_2. Subcell Biochem. 2014. PMID: 24962879 Review.
-
Nuclear and extra-nuclear effects of retinoid acid receptors: how they are interconnected.Subcell Biochem. 2014;70:103-27. doi: 10.1007/978-94-017-9050-5_6. Subcell Biochem. 2014. PMID: 24962883 Review.
-
The roles of retinoic acid and retinoic acid receptors in inducing epigenetic changes.Subcell Biochem. 2014;70:129-49. doi: 10.1007/978-94-017-9050-5_7. Subcell Biochem. 2014. PMID: 24962884 Free PMC article. Review.
-
LXR, a nuclear receptor that defines a distinct retinoid response pathway.Genes Dev. 1995 May 1;9(9):1033-45. doi: 10.1101/gad.9.9.1033. Genes Dev. 1995. PMID: 7744246
Cited by
-
Retinoid X Receptor Agonists Upregulate Genes Responsible for the Biosynthesis of All-Trans-Retinoic Acid in Human Epidermis.PLoS One. 2016 Apr 14;11(4):e0153556. doi: 10.1371/journal.pone.0153556. eCollection 2016. PLoS One. 2016. PMID: 27078158 Free PMC article.
-
Rexinoids Modulate Effector T Cell Expression of Mucosal Homing Markers CCR9 and α4β7 Integrin and Direct Their Migration In Vitro.Front Immunol. 2022 Jan 27;13:746484. doi: 10.3389/fimmu.2022.746484. eCollection 2022. Front Immunol. 2022. PMID: 35154092 Free PMC article.
-
The immunomodulatory role of all-trans retinoic acid in tumor microenvironment.Clin Exp Med. 2023 Jul;23(3):591-606. doi: 10.1007/s10238-022-00860-x. Epub 2022 Jul 13. Clin Exp Med. 2023. PMID: 35829844 Review.
-
Retinoic Acid: A New Old Friend of IL-17A in the Immune Pathogeny of Liver Fibrosis.Front Immunol. 2021 Jun 15;12:691073. doi: 10.3389/fimmu.2021.691073. eCollection 2021. Front Immunol. 2021. PMID: 34211477 Free PMC article. Review.
-
Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD).Nutrients. 2017 Dec 29;10(1):29. doi: 10.3390/nu10010029. Nutrients. 2017. PMID: 29286303 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

