SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress
- PMID: 24344202
- PMCID: PMC4023816
- DOI: 10.1128/MCB.01483-13
SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress
Abstract
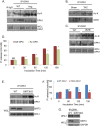
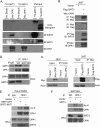
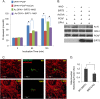
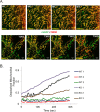
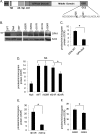
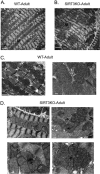
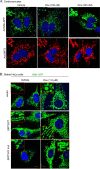
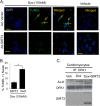
Mitochondrial morphology is regulated by the balance between two counteracting mitochondrial processes of fusion and fission. There is significant evidence suggesting a stringent association between morphology and bioenergetics of mitochondria. Morphological alterations in mitochondria are linked to several pathological disorders, including cardiovascular diseases. The consequences of stress-induced acetylation of mitochondrial proteins on the organelle morphology remain largely unexplored. Here we report that OPA1, a mitochondrial fusion protein, was hyperacetylated in hearts under pathological stress and this posttranslational modification reduced the GTPase activity of the protein. The mitochondrial deacetylase SIRT3 was capable of deacetylating OPA1 and elevating its GTPase activity. Mass spectrometry and mutagenesis analyses indicated that in SIRT3-deficient cells OPA1 was acetylated at lysine 926 and 931 residues. Overexpression of a deacetylation-mimetic version of OPA1 recovered the mitochondrial functions of OPA1-null cells, thus demonstrating the functional significance of K926/931 acetylation in regulating OPA1 activity. Moreover, SIRT3-dependent activation of OPA1 contributed to the preservation of mitochondrial networking and protection of cardiomyocytes from doxorubicin-mediated cell death. In summary, these data indicated that SIRT3 promotes mitochondrial function not only by regulating activity of metabolic enzymes, as previously reported, but also by regulating mitochondrial dynamics by _targeting OPA1.
Figures
Similar articles
-
Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development.Nat Commun. 2021 Jul 16;12(1):4371. doi: 10.1038/s41467-021-24619-2. Nat Commun. 2021. PMID: 34272364 Free PMC article.
-
Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway.J Cell Physiol. 2019 Dec;234(12):23495-23506. doi: 10.1002/jcp.28918. Epub 2019 Jun 7. J Cell Physiol. 2019. PMID: 31173361
-
SIRT3 promotes metabolic maturation of human iPSC-derived cardiomyocytes via OPA1-controlled mitochondrial dynamics.Free Radic Biol Med. 2023 Feb 1;195:270-282. doi: 10.1016/j.freeradbiomed.2022.12.101. Epub 2022 Dec 31. Free Radic Biol Med. 2023. PMID: 36596388
-
New insights into the function and regulation of mitochondrial fission.Biochim Biophys Acta. 2013 May;1833(5):1256-68. doi: 10.1016/j.bbamcr.2013.02.002. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434681 Review.
-
A link between protein acetylation and mitochondrial dynamics under energy metabolism: A comprehensive overview.J Cell Physiol. 2021 Dec;236(12):7926-7937. doi: 10.1002/jcp.30461. Epub 2021 Jun 8. J Cell Physiol. 2021. PMID: 34101176 Review.
Cited by
-
SIRT3 as a Regulator of Non-alcoholic Fatty Liver Disease.J Lifestyle Med. 2014 Sep;4(2):80-5. doi: 10.15280/jlm.2014.4.2.80. Epub 2014 Sep 30. J Lifestyle Med. 2014. PMID: 26064858 Free PMC article. Review.
-
Sirtuin3 ensures the metabolic plasticity of neurotransmission during glucose deprivation.J Cell Biol. 2024 Jan 1;223(1):e202305048. doi: 10.1083/jcb.202305048. Epub 2023 Nov 21. J Cell Biol. 2024. PMID: 37988067 Free PMC article.
-
Dexamethasone promotes mesenchymal stem cell apoptosis and inhibits osteogenesis by disrupting mitochondrial dynamics.FEBS Open Bio. 2020 Feb;10(2):211-220. doi: 10.1002/2211-5463.12771. Epub 2019 Dec 30. FEBS Open Bio. 2020. PMID: 31788976 Free PMC article.
-
Causal roles of mitochondrial dynamics in longevity and healthy aging.EMBO Rep. 2019 Dec 5;20(12):e48395. doi: 10.15252/embr.201948395. Epub 2019 Oct 31. EMBO Rep. 2019. PMID: 31667999 Free PMC article. Review.
-
Astragaloside IV Alleviates Infarction Induced Cardiomyocyte Injury by Improving Mitochondrial Morphology and Function.Front Cardiovasc Med. 2022 Feb 21;9:810541. doi: 10.3389/fcvm.2022.810541. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35265681 Free PMC article.
References
-
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. 2007. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27:8807–8814. 10.1128/MCB.01636-07 - DOI - PMC - PubMed
-
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. 2010. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17:41–52. 10.1016/j.ccr.2009.11.023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
