Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study
- PMID: 24345305
- PMCID: PMC3878731
- DOI: 10.1186/1471-2431-13-207
Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study
Abstract
Background: Bronchopulmonary dysplasia (BPD) is a common complication of preterm birth. Very different models using clinical parameters at an early postnatal age to predict BPD have been developed with little extensive quantitative validation. The objective of this study is to review and validate clinical prediction models for BPD.
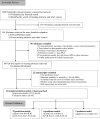
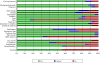
Methods: We searched the main electronic databases and abstracts from annual meetings. The STROBE instrument was used to assess the methodological quality. External validation of the retrieved models was performed using an individual patient dataset of 3229 patients at risk for BPD. Receiver operating characteristic curves were used to assess discrimination for each model by calculating the area under the curve (AUC). Calibration was assessed for the best discriminating models by visually comparing predicted and observed BPD probabilities.
Results: We identified 26 clinical prediction models for BPD. Although the STROBE instrument judged the quality from moderate to excellent, only four models utilised external validation and none presented calibration of the predictive value. For 19 prediction models with variables matched to our dataset, the AUCs ranged from 0.50 to 0.76 for the outcome BPD. Only two of the five best discriminating models showed good calibration.
Conclusions: External validation demonstrates that, except for two promising models, most existing clinical prediction models are poor to moderate predictors for BPD. To improve the predictive accuracy and identify preterm infants for future intervention studies aiming to reduce the risk of BPD, additional variables are required. Subsequently, that model should be externally validated using a proper impact analysis before its clinical implementation.
Figures





Similar articles
-
Bronchopulmonary dysplasia prediction models: a systematic review and meta-analysis with validation.Pediatr Res. 2023 Jul;94(1):43-54. doi: 10.1038/s41390-022-02451-8. Epub 2023 Jan 9. Pediatr Res. 2023. PMID: 36624282 Free PMC article.
-
The Prediction of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants through Clinical Indicators within 1 Hour of Delivery.J Korean Med Sci. 2021 Mar 22;36(11):e81. doi: 10.3346/jkms.2021.36.e81. J Korean Med Sci. 2021. PMID: 33754511 Free PMC article.
-
Bronchopulmonary dysplasia: a predictive scoring system for very low birth weight infants. A diagnostic accuracy study with prospective data collection.Eur J Pediatr. 2021 Aug;180(8):2453-2461. doi: 10.1007/s00431-021-04045-8. Epub 2021 Apr 6. Eur J Pediatr. 2021. PMID: 33822247 Free PMC article.
-
Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis.JAMA. 2016 Aug 9;316(6):611-24. doi: 10.1001/jama.2016.10708. JAMA. 2016. PMID: 27532916 Review.
-
Predictors of bronchopulmonary dysplasia.Clin Perinatol. 2012 Sep;39(3):585-601. doi: 10.1016/j.clp.2012.06.014. Clin Perinatol. 2012. PMID: 22954271 Free PMC article. Review.
Cited by
-
Early Neonatal Oxygen Exposure Predicts Pulmonary Morbidity and Functional Deficits at 1 Year.J Pediatr. 2020 Aug;223:20-28.e2. doi: 10.1016/j.jpeds.2020.04.042. J Pediatr. 2020. PMID: 32711747 Free PMC article.
-
Systemic Steroids in Preventing Bronchopulmonary Dysplasia (BPD): Neurodevelopmental Outcome According to the Risk of BPD in the EPICE Cohort.Int J Environ Res Public Health. 2022 May 5;19(9):5600. doi: 10.3390/ijerph19095600. Int J Environ Res Public Health. 2022. PMID: 35564997 Free PMC article.
-
Comparison of Multivariable Logistic Regression and Machine Learning Models for Predicting Bronchopulmonary Dysplasia or Death in Very Preterm Infants.Front Pediatr. 2021 Dec 7;9:759776. doi: 10.3389/fped.2021.759776. eCollection 2021. Front Pediatr. 2021. PMID: 34950616 Free PMC article.
-
Early prediction of pulmonary outcomes in preterm infants using electrical impedance tomography.Front Pediatr. 2023 May 24;11:1167077. doi: 10.3389/fped.2023.1167077. eCollection 2023. Front Pediatr. 2023. PMID: 37292377 Free PMC article.
-
Bronchopulmonary dysplasia prediction models: a systematic review and meta-analysis with validation.Pediatr Res. 2023 Jul;94(1):43-54. doi: 10.1038/s41390-022-02451-8. Epub 2023 Jan 9. Pediatr Res. 2023. PMID: 36624282 Free PMC article.
References
-
- Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, Wright LL, Ehrenkranz RA, Stoll BJ, Fanaroff AA. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;13(6):798–804. doi: 10.1016/j.jpeds.2005.01.047. - DOI - PubMed
-
- Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, Eisengart S, Baley J, Kercsmar C, Min MO. et al.Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;13(11):1082–1087. doi: 10.1001/archpedi.161.11.1082. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

