FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis
- PMID: 24509850
- PMCID: PMC3975025
- DOI: 10.1074/jbc.M113.527150
FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis
Abstract
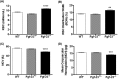
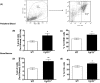
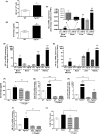
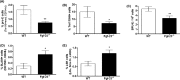
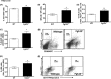
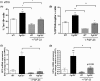
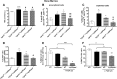
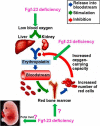
Abnormal blood cell production is associated with chronic kidney disease (CKD) and cardiovascular disease (CVD). Bone-derived FGF-23 (fibroblast growth factor-23) regulates phosphate homeostasis and bone mineralization. Genetic deletion of Fgf-23 in mice (Fgf-23(-/-)) results in hypervitaminosis D, abnormal mineral metabolism, and reduced lymphatic organ size. Elevated FGF-23 levels are linked to CKD and greater risk of CVD, left ventricular hypertrophy, and mortality in dialysis patients. However, whether FGF-23 is involved in the regulation of erythropoiesis is unknown. Here we report that loss of FGF-23 results in increased hematopoietic stem cell frequency associated with increased erythropoiesis in peripheral blood and bone marrow in young adult mice. In particular, these hematopoietic changes are also detected in fetal livers, suggesting that they are not the result of altered bone marrow niche alone. Most importantly, administration of FGF-23 in wild-type mice results in a rapid decrease in erythropoiesis. Finally, we show that the effect of FGF-23 on erythropoiesis is independent of the high vitamin D levels in these mice. Our studies suggest a novel role for FGF-23 in erythrocyte production and differentiation and suggest that elevated FGF-23 levels contribute to the pathogenesis of anemia in patients with CKD and CVD.
Keywords: Anemia; Bone; Bone Marrow; Erythrocyte; Erythropoeisis; FGF-23; Hematopoiesis; Kidney.
Figures





Similar articles
-
Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis.Am J Pathol. 2014 Mar;184(3):827-41. doi: 10.1016/j.ajpath.2013.11.016. Epub 2014 Jan 8. Am J Pathol. 2014. PMID: 24412515 Free PMC article.
-
Fibroblast growth factor 23: associations with cardiovascular disease and mortality in chronic kidney disease.Int Urol Nephrol. 2014 Jan;46(1):9-17. doi: 10.1007/s11255-012-0370-2. Epub 2013 Jan 8. Int Urol Nephrol. 2014. PMID: 23296792 Review.
-
The secreted lymphangiogenic factor CCBE1 is essential for fetal liver erythropoiesis.Blood. 2013 Apr 18;121(16):3228-36. doi: 10.1182/blood-2012-10-462689. Epub 2013 Feb 20. Blood. 2013. PMID: 23426945 Free PMC article.
-
The role of fibroblast growth factor 23 in chronic kidney disease-mineral and bone disorder.Nefrologia. 2013 Nov 13;33(6):835-44. doi: 10.3265/Nefrologia.pre2013.Jul.12091. Epub 2013 Oct 25. Nefrologia. 2013. PMID: 24158124 Review.
-
Fibroblast growth factor-23 helps explain the biphasic cardiovascular effects of vitamin D in chronic kidney disease.Int J Biol Sci. 2012;8(5):663-71. doi: 10.7150/ijbs.3886. Epub 2012 May 5. Int J Biol Sci. 2012. PMID: 22606047 Free PMC article. Review.
Cited by
-
Effects of erythropoietin on fibroblast growth factor 23 in mice and humans.Nephrol Dial Transplant. 2019 Dec 1;34(12):2057-2065. doi: 10.1093/ndt/gfy189. Nephrol Dial Transplant. 2019. PMID: 30007314 Free PMC article.
-
New concepts in regulation and function of the FGF23.Clin Exp Med. 2023 Aug;23(4):1055-1066. doi: 10.1007/s10238-022-00844-x. Epub 2022 Jun 16. Clin Exp Med. 2023. PMID: 35708778 Review.
-
Peripheral Blood Mononuclear Cells (PBMCs) to Dissect the Underlying Mechanisms of Bone Disease in Chronic Kidney Disease and Rare Renal Diseases.Curr Osteoporos Rep. 2021 Dec;19(6):553-562. doi: 10.1007/s11914-021-00707-6. Epub 2021 Nov 13. Curr Osteoporos Rep. 2021. PMID: 34773213 Review.
-
Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease.Sci Rep. 2018 May 8;8(1):7294. doi: 10.1038/s41598-018-25439-z. Sci Rep. 2018. PMID: 29740119 Free PMC article.
-
Associations between anemia and FGF23 in the CKiD study.Pediatr Nephrol. 2024 Mar;39(3):837-847. doi: 10.1007/s00467-023-06160-0. Epub 2023 Sep 26. Pediatr Nephrol. 2024. PMID: 37752381 Free PMC article.
References
-
- Lipkin G. W., Kendall R. G., Russon L. J., Turney J. H., Norfolk D. R., Brownjohn A. M. (1990) Erythropoietin deficiency in acute renal failure. Nephrol. Dial. Transplant. 5, 920–922 - PubMed
-
- Zhang F., Laneuville P., Gagnon R. F., Morin B., Brox A. G. (1996) Effect of chronic renal failure on the expression of erythropoietin message in a murine model. Exp. Hematol. 24, 1469–1474 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

