Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells
- PMID: 24551118
- PMCID: PMC3925144
- DOI: 10.1371/journal.pone.0088556
Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells
Abstract
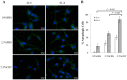
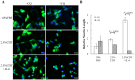
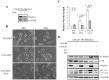
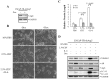
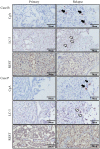
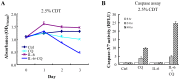
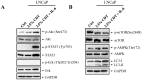
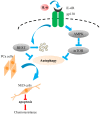
Prostate cancer (PCa) cells undergoing neuroendocrine differentiation (NED) are clinically relevant to the development of relapsed castration-resistant PCa. Increasing evidences show that autophagy involves in the development of neuroendocrine (NE) tumors, including PCa. To clarify the effect of autophagy on NED, androgen-sensitive PCa LNCaP cells were examined. Treatment of LNCaP cells with IL-6 resulted in an induction of autophagy. In the absence of androgen, IL-6 caused an even stronger activation of autophagy. Similar result was identified in NED induction. Inhibition of autophagy with chloroquine (CQ) markedly decreased NED. This observation was confirmed by beclin1 and Atg5 silencing experiments. Further supporting the role of autophagy in NED, we found that LC3 was up-regulated in PCa tissue that had relapsed after androgen-deprivation therapy when compared with their primary tumor counterpart. LC3 staining in relapsed PCa tissue showed punctate pattern similar to the staining of chromogranin A (CgA), a marker for NED cells. Moreover, autophagy inhibition induced the apoptosis of IL-6 induced NE differentiated PCa cells. Consistently, inhibition of autophagy by knockdown of beclin1 or Atg5 sensitized NE differentiated LNCaP cells to etoposide, a chemotherapy drug. To identify the mechanisms, phosphorylation of IL-6 downstream _targets was analyzed. An increase in phospho-AMPK and a decrease in phospho-mTOR were found, which implies that IL-6 regulates autophagy through the AMPK/mTOR pathway. Most important to this study is the discovery of REST, a neuronal gene-specific transcriptional repressor that is involved in autophagy activation. REST was down-regulated in IL-6 treatment. Knockdown experiments suggest that REST is critical to NED and autophagy activation by IL-6. Together, our studies imply that autophagy is involved in PCa progression and plays a cytoprotective role when NED is induced in PCa cells by IL-6 treatment. These results reveal the potential of _targeting autophagy as part of a combined therapeutic regime for NE tumors.
Conflict of interest statement
Figures



Similar articles
-
REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling.Onco_target. 2016 May 3;7(18):26137-51. doi: 10.18632/onco_target.8433. Onco_target. 2016. PMID: 27034167 Free PMC article.
-
Interleukin-6: a bone marrow stromal cell paracrine signal that induces neuroendocrine differentiation and modulates autophagy in bone metastatic PCa cells.Autophagy. 2012 Apr;8(4):650-63. doi: 10.4161/auto.19226. Epub 2012 Apr 1. Autophagy. 2012. PMID: 22441019 Free PMC article.
-
Interleukin-6 induces neuroendocrine differentiation (NED) through suppression of RE-1 silencing transcription factor (REST).Prostate. 2014 Aug;74(11):1086-94. doi: 10.1002/pros.22819. Epub 2014 May 12. Prostate. 2014. PMID: 24819501
-
Neuroendocrine differentiation in the progression of prostate cancer.Int J Urol. 2009 Jan;16(1):37-44. doi: 10.1111/j.1442-2042.2008.02175.x. Int J Urol. 2009. PMID: 19120524 Review.
-
ADT with antiandrogens in prostate cancer induces adverse effect of increasing resistance, neuroendocrine differentiation and tumor metastasis.Cancer Lett. 2018 Dec 28;439:47-55. doi: 10.1016/j.canlet.2018.09.020. Epub 2018 Sep 15. Cancer Lett. 2018. PMID: 30227222 Review.
Cited by
-
Autophagy requires poly(adp-ribosyl)ation-dependent AMPK nuclear export.Cell Death Differ. 2016 Dec;23(12):2007-2018. doi: 10.1038/cdd.2016.80. Epub 2016 Sep 30. Cell Death Differ. 2016. PMID: 27689873 Free PMC article.
-
The regulatory pathways leading to stem-like cells underlie prostate cancer progression.Asian J Androl. 2019 May-Jun;21(3):233-240. doi: 10.4103/aja.aja_72_18. Asian J Androl. 2019. PMID: 30178777 Free PMC article. Review.
-
Key players of neuroendocrine differentiation in prostate cancer.Ann Transl Med. 2019 Jul;7(Suppl 3):S112. doi: 10.21037/atm.2019.05.18. Ann Transl Med. 2019. PMID: 31576319 Free PMC article. No abstract available.
-
TNFAIP8 promotes prostate cancer cell survival by inducing autophagy.Onco_target. 2018 Jun 1;9(42):26884-26899. doi: 10.18632/onco_target.25529. eCollection 2018 Jun 1. Onco_target. 2018. PMID: 29928491 Free PMC article.
-
Cellular rewiring in lethal prostate cancer: the architect of drug resistance.Nat Rev Urol. 2020 May;17(5):292-307. doi: 10.1038/s41585-020-0298-8. Epub 2020 Mar 16. Nat Rev Urol. 2020. PMID: 32203305 Free PMC article. Review.
References
-
- Sim HG, Cheng CW (2005) Changing demography of prostate cancer in Asia. Eur J Cancer 41: 834–845. - PubMed
-
- Isaacs JT (1984) The timing of androgen ablation therapy and/or chemotherapy in the treatment of prostatic cancer. Prostate 5: 1–17. - PubMed
-
- Marcu M, Radu E, Sajin M (2010) Neuroendocrine transdifferentiation of prostate carcinoma cells and its prognostic significance. Rom J Morphol Embryol 51: 7–12. - PubMed
-
- Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, et al. (2009) Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 16: 37–44. - PubMed
-
- Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N (2004) Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol 45: 586–592 discussion 592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

