Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia
- PMID: 24560881
- PMCID: PMC4151500
- DOI: 10.1016/j.schres.2014.01.028
Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia
Abstract
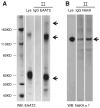
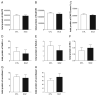
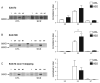
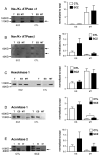
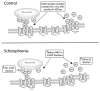
Excitatory amino acid transporter 2 (EAAT2) belongs to a family of Na(+) dependent glutamate transporters that maintain a low synaptic concentration of glutamate by removing glutamate from the synaptic cleft into astroglia and neurons. EAAT2 activity depends on Na(+) and K(+) gradients generated by Na(+)/K(+) ATPase and ATP. Hexokinase 1 (HK1), an initial enzyme of glycolysis, binds to mitochondrial outer membrane where it couples cytosolic glycolysis to mitochondrial oxidative phosphorylation, producing ATP utilized by the EAAT2/Na(+)/K(+) ATPase protein complex to facilitate glutamate reuptake. In this study, we hypothesized that the protein complex formed by EAAT2, Na(+)/K(+) ATPase and mitochondrial proteins in human postmortem prefrontal cortex may be disrupted, leading to abnormal glutamate transmission in schizophrenia. We first determined that EAAT2, Na(+)/K(+) ATPase, HK1 and aconitase were found in both EAAT2 and Na(+)/K(+) ATPase interactomes by immunoisolation and mass spectrometry in human postmortem prefrontal cortex. Next, we measured levels of glutamate transport complex proteins in subcellular fractions in the dorsolateral prefrontal cortex and found increases in the EAAT2B isoform of EAAT2 in a fraction containing extrasynaptic membranes and increased aconitase 1 in a mitochondrial fraction. Finally, an increased ratio of HK1 protein in the extrasynaptic membrane/mitochondrial fraction was found in subjects with schizophrenia, suggesting that HK1 protein is abnormally partitioned in this illness. Our findings indicate that the integrity of the glutamate transport protein complex may be disrupted, leading to decreased perisynaptic buffering and reuptake of glutamate, as well as impaired energy metabolism in schizophrenia.
Keywords: Dorsolateral prefrontal cortex (DLPFC); Excitatory amino acid transporter (EAAT); Immunoisolation; Mass spectrometry; Postmortem; Reuptake.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


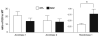
Similar articles
-
Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: a light and electron microscopic study.Neuroscience. 2014 Sep 26;277:522-40. doi: 10.1016/j.neuroscience.2014.07.019. Epub 2014 Jul 24. Neuroscience. 2014. PMID: 25064059 Free PMC article.
-
Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia.Transl Psychiatry. 2015 Jun 9;5(6):e579. doi: 10.1038/tp.2015.74. Transl Psychiatry. 2015. PMID: 26057049 Free PMC article.
-
Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia.Schizophr Res. 2010 Mar;117(1):92-8. doi: 10.1016/j.schres.2009.07.025. Epub 2009 Aug 27. Schizophr Res. 2010. PMID: 19716271 Free PMC article.
-
Glutamatergic hypothesis of schizophrenia: involvement of Na+/K+-dependent glutamate transport.J Biomed Sci. 2005 Dec;12(6):975-84. doi: 10.1007/s11373-005-9015-0. Epub 2005 Oct 14. J Biomed Sci. 2005. PMID: 16228297 Review.
-
Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death.Arch Med Res. 2006 Jan;37(1):11-8. doi: 10.1016/j.arcmed.2005.05.014. Arch Med Res. 2006. PMID: 16314180 Review.
Cited by
-
Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia.Schizophr Res. 2015 Aug;166(1-3):219-24. doi: 10.1016/j.schres.2015.06.002. Epub 2015 Jun 20. Schizophr Res. 2015. PMID: 26104473 Free PMC article.
-
Displacing hexokinase from mitochondrial voltage-dependent anion channel impairs GLT-1-mediated glutamate uptake but does not disrupt interactions between GLT-1 and mitochondrial proteins.J Neurosci Res. 2015 Jul;93(7):999-1008. doi: 10.1002/jnr.23533. Epub 2014 Dec 26. J Neurosci Res. 2015. PMID: 25546576 Free PMC article.
-
Decreased chloride channel expression in the dorsolateral prefrontal cortex in schizophrenia.PLoS One. 2015 Mar 31;10(3):e0123158. doi: 10.1371/journal.pone.0123158. eCollection 2015. PLoS One. 2015. PMID: 25826365 Free PMC article.
-
Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain.Transl Psychiatry. 2015 Aug 4;5(8):e612. doi: 10.1038/tp.2015.102. Transl Psychiatry. 2015. PMID: 26241350 Free PMC article.
-
The Influence of Na(+), K(+)-ATPase on Glutamate Signaling in Neurodegenerative Diseases and Senescence.Front Physiol. 2016 Jun 2;7:195. doi: 10.3389/fphys.2016.00195. eCollection 2016. Front Physiol. 2016. PMID: 27313535 Free PMC article. Review.
References
-
- Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem. 2008;283(19):13482–13490. - PubMed
-
- Balaban RS, Bader JP. Studies on the relationship between glycolysis and (Na+ + K+)-ATPase in cultured cells. Biochim Biophys Acta. 1984;804(4):419–426. - PubMed
-
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. J Neurochem. 1997;69(6):2571–2580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH053327/MH/NIMH NIH HHS/United States
- MH064673/MH/NIMH NIH HHS/United States
- MH074016/MH/NIMH NIH HHS/United States
- R01 MH064673/MH/NIMH NIH HHS/United States
- K08 MH074016/MH/NIMH NIH HHS/United States
- MH066392/MH/NIMH NIH HHS/United States
- RC1 MH088752/MH/NIMH NIH HHS/United States
- R01 MH094445/MH/NIMH NIH HHS/United States
- MH53327/MH/NIMH NIH HHS/United States
- MH88752/MH/NIMH NIH HHS/United States
- R21 MH087752/MH/NIMH NIH HHS/United States
- P50 MH066392/MH/NIMH NIH HHS/United States
- MH094445/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

