A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy
- PMID: 24565221
- PMCID: PMC3946011
- DOI: 10.1186/1472-6823-14-17
A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy
Abstract
Background: To compare the safety and efficacy of saxagliptin 2.5 mg twice daily (BID) versus placebo add-on therapy to metformin immediate release (IR) in patients with type 2 diabetes and inadequate glycemic control with metformin alone.
Methods: This multicenter, 12-week, double-blind, parallel-group trial enrolled adult outpatients with type 2 diabetes (glycated hemoglobin [HbA1c] 7.0%-10.0%) on stable metformin IR monotherapy (≥1500 mg, BID for ≥8 weeks). Patients were randomized to double-blind saxagliptin 2.5 mg BID or placebo added on to metformin IR following a 2-week, single-blind, placebo add-on therapy lead-in period. The primary end point was the change from baseline to week 12 in HbA1c. Key secondary end points included change from baseline to week 12 in fasting plasma glucose (FPG) and the proportion of patients achieving HbA1c <7.0% or HbA1c ≤ 6.5% at week 12. Efficacy was analyzed in all patients who received randomized study drug with ≥1 postbaseline assessment. Safety was assessed in all treated patients.
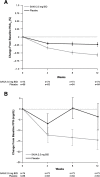
Results: In total, 74 patients were randomized to double-blind saxagliptin add-on therapy and 86 to placebo add-on therapy. At week 12, least-squares mean changes (95% CI) from baseline HbA1c (adjusted for baseline HbA1c) were significantly greater (P = 0.006) in the saxagliptin + metformin group -0.56% (-0.74% to -0.38%) versus the placebo + metformin group -0.22% (-0.39% to -0.06%). Adjusted mean changes from baseline in FPG were numerically greater with saxagliptin versus placebo; the difference (95% CI) -9.5 mg/dL (-21.7 to 2.7) was not statistically significant (P = 0.12). A numerically greater proportion of patients in the saxagliptin group than the placebo group achieved HbA1c < 7.0% (37.5% vs 24.2%) or HbA1c ≤6.5% (24.6% vs 10.7%). There were no unexpected safety findings. Hypoglycemia occurred in 4 patients (5.4%) in the saxagliptin group and 1 patient (1.2%) in the placebo group; confirmed hypoglycemia (symptoms plus fingerstick glucose ≤50 mg/dL) occurred in 1 patient in the placebo group.
Conclusions: Addition of saxagliptin 2.5 mg BID to metformin therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy reduced HbA1c compared with placebo added to metformin, with an adverse events profile similar to placebo and no unexpected safety findings.
Trial registration: ClinicalTrials.gov NCT00885378.
Figures
Similar articles
-
Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy.Clin Drug Investig. 2013 Oct;33(10):707-17. doi: 10.1007/s40261-013-0107-8. Clin Drug Investig. 2013. PMID: 23949898 Clinical Trial.
-
Randomized, Double-Blind, Phase 3 Trial of Triple Therapy With Dapagliflozin Add-on to Saxagliptin Plus Metformin in Type 2 Diabetes.Diabetes Care. 2015 Nov;38(11):2009-17. doi: 10.2337/dc15-0779. Epub 2015 Aug 5. Diabetes Care. 2015. PMID: 26246458 Clinical Trial.
-
Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial.Diabetes Obes Metab. 2009 Jun;11(6):611-22. doi: 10.1111/j.1463-1326.2009.01056.x. Diabetes Obes Metab. 2009. PMID: 19515181 Clinical Trial.
-
Profile of saxagliptin in the treatment of type 2 diabetes: focus on Japanese patients.Ther Clin Risk Manag. 2014 Jul 11;10:547-58. doi: 10.2147/TCRM.S46076. eCollection 2014. Ther Clin Risk Manag. 2014. PMID: 25050065 Free PMC article. Review.
-
Comparison of the Efficacy and Safety of Metformin-Based Combination Therapy Versus Metformin Alone in Children and Adolescents With Type 2 Diabetes Mellitus: A Meta-Analysis.Cureus. 2023 Feb 15;15(2):e35014. doi: 10.7759/cureus.35014. eCollection 2023 Feb. Cureus. 2023. PMID: 36938239 Free PMC article. Review.
Cited by
-
Overview of saxagliptin efficacy and safety in patients with type 2 diabetes and cardiovascular disease or risk factors for cardiovascular disease.Vasc Health Risk Manag. 2014 Dec 23;11:9-23. doi: 10.2147/VHRM.S75215. eCollection 2015. Vasc Health Risk Manag. 2014. PMID: 25565858 Free PMC article. Review.
-
Cardiovascular Effect of Incretin-Based Therapy in Patients with Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis.PLoS One. 2016 Apr 14;11(4):e0153502. doi: 10.1371/journal.pone.0153502. eCollection 2016. PLoS One. 2016. PMID: 27078018 Free PMC article. Review.
-
Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: pooled analysis of 20 clinical trials.Cardiovasc Diabetol. 2014 Feb 4;13:33. doi: 10.1186/1475-2840-13-33. Cardiovasc Diabetol. 2014. PMID: 24490835 Free PMC article.
-
Factors Related to the Glucose-Lowering Efficacy of Dipeptidyl Peptidase-4 Inhibitors: A Systematic Review and Meta-Analysis Focusing on Ethnicity and Study Regions.Clin Drug Investig. 2017 Mar;37(3):219-232. doi: 10.1007/s40261-016-0478-8. Clin Drug Investig. 2017. PMID: 27848150 Review.
-
Assessment of Saxagliptin Efficacy: Meta-Analysis of 14 Phase 2 and 3 Clinical Trials.Diabetes Ther. 2017 Jun;8(3):587-599. doi: 10.1007/s13300-017-0261-8. Epub 2017 Apr 21. Diabetes Ther. 2017. PMID: 28432619 Free PMC article.
References
-
- Zinman B. Initial combination therapy for type 2 diabetes mellitus: is it ready for prime time? Am J Med. 2011;124(1 Suppl):S19–S34. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. - DOI - PMC - PubMed
-
- Global guideline for type 2 diabetes. http://www.staff.ncl.ac.uk/philip.home/IDF%20GGT2D.pdf.
-
- Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda O, Garber AJ, Hirsch IB, Horton ES, Ismail-Beigi F, Jellinger PS, Jones KL, Jovanovič L, Lebovitz H, Levy P, Moghissi ET, Orzeck EA, Vinik AI, Wyne KL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(2 Suppl):1–53. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

