Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys
- PMID: 24647955
- PMCID: PMC3960473
- DOI: 10.1523/JNEUROSCI.4954-13.2014
Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys
Abstract
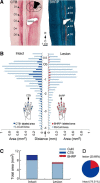
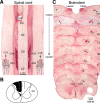
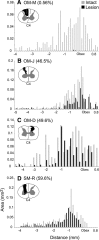
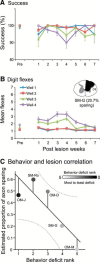
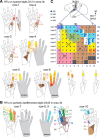
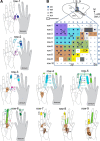
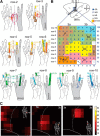
Lesions of the dorsal columns at a mid-cervical level render the hand representation of the contralateral primary somatosensory cortex (area 3b) unresponsive. Over weeks of recovery, most of this cortex becomes responsive to touch on the hand. Determining functional properties of neurons within the hand representation is critical to understanding the neural basis of this adaptive plasticity. Here, we recorded neural activity across the hand representation of area 3b with a 100-electrode array and compared results from owl monkeys and squirrel monkeys 5-10 weeks after lesions with controls. Even after extensive lesions, performance on reach-to-grasp tasks returned to prelesion levels, and hand touches activated territories mainly within expected cortical locations. However, some digit representations were abnormal, such that receptive fields of presumably reactivated neurons were larger and more often involved discontinuous parts of the hand compared with controls. Hand stimulation evoked similar neuronal firing rates in lesion and control monkeys. By assessing the same monkeys with multiple measures, we determined that properties of neurons in area 3b were highly correlated with both the lesion severity and the impairment of hand use. We propose that the reactivation of neurons with near-normal response properties and the recovery of near-normal somatotopy likely supported the recovery of hand use. Given the near-completeness of the more extensive dorsal column lesions we studied, we suggest that alternate spinal afferents, in addition to the few spared primary axon afferents in the dorsal columns, likely have a major role in the reactivation pattern and return of function.
Keywords: area 3b; dorsal column lesion; multielectrode array; primate; somatotopy; tactile.
Figures







Similar articles
-
Spinal cord neuron inputs to the cuneate nucleus that partially survive dorsal column lesions: A pathway that could contribute to recovery after spinal cord injury.J Comp Neurol. 2015 Oct 1;523(14):2138-60. doi: 10.1002/cne.23783. Epub 2015 Jun 2. J Comp Neurol. 2015. PMID: 25845707 Free PMC article.
-
Longitudinal fMRI measures of cortical reactivation and hand use with and without training after sensory loss in primates.Neuroimage. 2021 Aug 1;236:118026. doi: 10.1016/j.neuroimage.2021.118026. Epub 2021 Apr 27. Neuroimage. 2021. PMID: 33930537 Free PMC article.
-
Second-order spinal cord pathway contributes to cortical responses after long recoveries from dorsal column injury in squirrel monkeys.Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4258-4263. doi: 10.1073/pnas.1718826115. Epub 2018 Apr 2. Proc Natl Acad Sci U S A. 2018. PMID: 29610299 Free PMC article.
-
Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord.Exp Neurol. 2008 Feb;209(2):407-16. doi: 10.1016/j.expneurol.2007.06.014. Epub 2007 Jul 6. Exp Neurol. 2008. PMID: 17692844 Free PMC article. Review.
-
The reactivation of somatosensory cortex and behavioral recovery after sensory loss in mature primates.Front Syst Neurosci. 2014 May 12;8:84. doi: 10.3389/fnsys.2014.00084. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24860443 Free PMC article. Review.
Cited by
-
Spinal cord neuron inputs to the cuneate nucleus that partially survive dorsal column lesions: A pathway that could contribute to recovery after spinal cord injury.J Comp Neurol. 2015 Oct 1;523(14):2138-60. doi: 10.1002/cne.23783. Epub 2015 Jun 2. J Comp Neurol. 2015. PMID: 25845707 Free PMC article.
-
Longitudinal fMRI measures of cortical reactivation and hand use with and without training after sensory loss in primates.Neuroimage. 2021 Aug 1;236:118026. doi: 10.1016/j.neuroimage.2021.118026. Epub 2021 Apr 27. Neuroimage. 2021. PMID: 33930537 Free PMC article.
-
Congenital foot deformation alters the topographic organization in the primate somatosensory system.Brain Struct Funct. 2016 Jan;221(1):383-406. doi: 10.1007/s00429-014-0913-7. Epub 2014 Oct 18. Brain Struct Funct. 2016. PMID: 25326245 Free PMC article.
-
Intracortical connections are altered after long-standing deprivation of dorsal column inputs in the hand region of area 3b in squirrel monkeys.J Comp Neurol. 2016 May 1;524(7):1494-526. doi: 10.1002/cne.23921. Epub 2015 Dec 8. J Comp Neurol. 2016. PMID: 26519356 Free PMC article.
-
Plasticity and Recovery After Dorsal Column Spinal Cord Injury in Nonhuman Primates.J Exp Neurosci. 2016 Aug 18;10(Suppl 1):11-21. doi: 10.4137/JEN.S40197. eCollection 2016. J Exp Neurosci. 2016. PMID: 27578996 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
