Local renal circadian clocks control fluid-electrolyte homeostasis and BP
- PMID: 24652800
- PMCID: PMC4073428
- DOI: 10.1681/ASN.2013060641
Local renal circadian clocks control fluid-electrolyte homeostasis and BP
Abstract
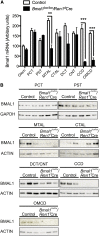
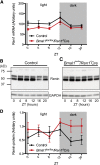
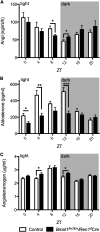
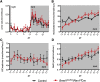
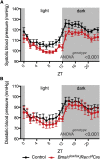
The circadian timing system is critically involved in the maintenance of fluid and electrolyte balance and BP control. However, the role of peripheral circadian clocks in these homeostatic mechanisms remains unknown. We addressed this question in a mouse model carrying a conditional allele of the circadian clock gene Bmal1 and expressing Cre recombinase under the endogenous Renin promoter (Bmal1(lox/lox)/Ren1(d)Cre mice). Analysis of Bmal1(lox/lox)/Ren1(d)Cre mice showed that the floxed Bmal1 allele was excised in the kidney. In the kidney, BMAL1 protein expression was absent in the renin-secreting granular cells of the juxtaglomerular apparatus and the collecting duct. A partial reduction of BMAL1 expression was observed in the medullary thick ascending limb. Functional analyses showed that Bmal1(lox/lox)/Ren1(d)Cre mice exhibited multiple abnormalities, including increased urine volume, changes in the circadian rhythm of urinary sodium excretion, increased GFR, and significantly reduced plasma aldosterone levels. These changes were accompanied by a reduction in BP. These results show that local renal circadian clocks control body fluid and BP homeostasis.
Copyright © 2014 by the American Society of Nephrology.
Figures


Comment in
-
Tick tock: time to recognize the kidney clock.J Am Soc Nephrol. 2014 Jul;25(7):1369-71. doi: 10.1681/ASN.2014020199. Epub 2014 Mar 20. J Am Soc Nephrol. 2014. PMID: 24652798 Free PMC article. No abstract available.
Similar articles
-
Adrenal-Specific KO of the Circadian Clock Protein BMAL1 Alters Blood Pressure Rhythm and Timing of Eating Behavior.Function (Oxf). 2023 Jan 9;4(2):zqad001. doi: 10.1093/function/zqad001. eCollection 2023. Function (Oxf). 2023. PMID: 36778748 Free PMC article.
-
Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition.J Am Soc Nephrol. 2016 Oct;27(10):2997-3004. doi: 10.1681/ASN.2015091055. Epub 2016 Apr 7. J Am Soc Nephrol. 2016. PMID: 27056296 Free PMC article.
-
Tick tock: time to recognize the kidney clock.J Am Soc Nephrol. 2014 Jul;25(7):1369-71. doi: 10.1681/ASN.2014020199. Epub 2014 Mar 20. J Am Soc Nephrol. 2014. PMID: 24652798 Free PMC article. No abstract available.
-
Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues.Neurochem Int. 2018 Oct;119:11-16. doi: 10.1016/j.neuint.2017.12.013. Epub 2018 Jan 3. Neurochem Int. 2018. PMID: 29305918 Review.
-
Role of circadian rhythms in potassium homeostasis.Semin Nephrol. 2013 May;33(3):229-36. doi: 10.1016/j.semnephrol.2013.04.003. Semin Nephrol. 2013. PMID: 23953800 Free PMC article. Review.
Cited by
-
Adrenal-Specific KO of the Circadian Clock Protein BMAL1 Alters Blood Pressure Rhythm and Timing of Eating Behavior.Function (Oxf). 2023 Jan 9;4(2):zqad001. doi: 10.1093/function/zqad001. eCollection 2023. Function (Oxf). 2023. PMID: 36778748 Free PMC article.
-
The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology.Eur J Pediatr. 2016 Jun;175(6):747-54. doi: 10.1007/s00431-016-2729-3. Epub 2016 May 2. Eur J Pediatr. 2016. PMID: 27138767 Review.
-
Circadian gene expression in mouse renal proximal tubule.Am J Physiol Renal Physiol. 2023 Mar 1;324(3):F301-F314. doi: 10.1152/ajprenal.00231.2022. Epub 2023 Feb 2. Am J Physiol Renal Physiol. 2023. PMID: 36727945 Free PMC article.
-
Autonomic nerves and circadian control of renal function.Auton Neurosci. 2019 Mar;217:58-65. doi: 10.1016/j.autneu.2019.01.003. Epub 2019 Jan 10. Auton Neurosci. 2019. PMID: 30704976 Free PMC article. Review.
-
RECENT ADVANCES IN UNDERSTANDING THE CIRCADIAN CLOCK IN RENAL PHYSIOLOGY.Curr Opin Physiol. 2018 Oct;5:38-44. doi: 10.1016/j.cophys.2018.06.002. Epub 2018 Jun 20. Curr Opin Physiol. 2018. PMID: 30714020 Free PMC article.
References
-
- Saini C, Suter DM, Liani A, Gos P, Schibler U: The mammalian circadian timing system: Synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol 76: 39–47, 2011 - PubMed
-
- Bass J: Circadian topology of metabolism. Nature 491: 348–356, 2012 - PubMed
-
- Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP: Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Physiol Regul Integr Comp Physiol 298: R627–R634, 2010 - PubMed
-
- Wang Q, Maillard M, Schibler U, Burnier M, Gachon F: Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am J Physiol Regul Integr Comp Physiol 299: R1013–R1019, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

