Cellular accumulation of Cys326-OGG1 protein complexes under conditions of oxidative stress
- PMID: 24680828
- PMCID: PMC4005915
- DOI: 10.1016/j.bbrc.2014.03.044
Cellular accumulation of Cys326-OGG1 protein complexes under conditions of oxidative stress
Abstract
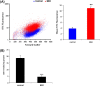
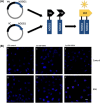
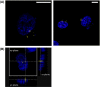
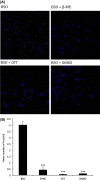
The common Ser326Cys polymorphism in the base excision repair protein 8-oxoguanine glycosylase 1 is associated with a reduced capacity to repair oxidative DNA damage particularly under conditions of intracellular oxidative stress and there is evidence that Cys326-OGG1 homozygous individuals have increased susceptibility to specific cancer types. Indirect biochemical studies have shown that reduced repair capacity is related to OGG1 redox modification and also possibly OGG1 dimer formation. In the current study we have used bimolecular fluorescence complementation to study for the first time a component of the base excision repair pathway and applied it to visualise accumulation of Cys326-OGG1 protein complexes in the native cellular environment. Fluorescence was observed both within and around the cell nucleus, was shown to be specific to cells expressing Cys326-OGG1 and only occurred in cells under conditions of cellular oxidative stress following depletion of intracellular glutathione levels by treatment with buthionine sulphoximine. Furthermore, OGG1 complex formation was inhibited by incubation of cells with the thiol reducing agents β-mercaptoethanol and dithiothreitol and the antioxidant dimethylsulfoxide indicating a causative role for oxidative stress in the formation of OGG1 cellular complexes. In conclusion, this study has provided for the first time evidence of redox sensitive Cys326-OGG1 protein accumulation in cells under conditions of intracellular oxidative stress that may be related to the previously reported reduced repair capacity of Cys326-OGG1 specifically under conditions of oxidative stress.
Keywords: BiFC visualisation; Dimer; OGG1; Oxidative stress.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells.DNA Repair (Amst). 2015 Feb;26:15-22. doi: 10.1016/j.dnarep.2014.11.007. Epub 2014 Dec 4. DNA Repair (Amst). 2015. PMID: 25534136 Free PMC article.
-
Repair of oxidative DNA damage is delayed in the Ser326Cys polymorphic variant of the base excision repair protein OGG1.Mutagenesis. 2012 Jul;27(4):501-10. doi: 10.1093/mutage/ges012. Epub 2012 Mar 25. Mutagenesis. 2012. PMID: 22451681
-
Direct visualization of repair of oxidative damage by OGG1 in the nuclei of live cells.J Biochem Mol Toxicol. 2011 Jan-Feb;25(1):1-7. doi: 10.1002/jbt.20346. Epub 2010 Nov 15. J Biochem Mol Toxicol. 2011. PMID: 21322094
-
Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome?Int J Mol Sci. 2020 Nov 7;21(21):8360. doi: 10.3390/ijms21218360. Int J Mol Sci. 2020. PMID: 33171795 Free PMC article. Review.
-
Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases.Free Radic Biol Med. 2017 Jun;107:179-201. doi: 10.1016/j.freeradbiomed.2016.11.042. Epub 2016 Nov 27. Free Radic Biol Med. 2017. PMID: 27903453 Review.
Cited by
-
Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells.DNA Repair (Amst). 2015 Feb;26:15-22. doi: 10.1016/j.dnarep.2014.11.007. Epub 2014 Dec 4. DNA Repair (Amst). 2015. PMID: 25534136 Free PMC article.
-
Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling.Free Radic Biol Med. 2015 Dec;89:369-78. doi: 10.1016/j.freeradbiomed.2015.08.015. Epub 2015 Sep 30. Free Radic Biol Med. 2015. PMID: 26431905 Free PMC article.
-
Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer.Pharmacol Ther. 2019 Feb;194:59-72. doi: 10.1016/j.pharmthera.2018.09.004. Epub 2018 Sep 19. Pharmacol Ther. 2019. PMID: 30240635 Free PMC article. Review.
References
-
- Lyng F., Seymour C., Mothersill C. Oxidative stress in cells exposed to low levels of ionizing radiation. Biochem. Soc. Trans. 2001;29:350–353. - PubMed
-
- Harris R., Williams T., Hodges N., Waring R. Reactive oxygen species and oxidative DNA damage mediate the cytotoxicity of tungsten–nickel–cobalt alloys in vitro. Toxicol. Appl. Pharmacol. 2011;250:19–28. - PubMed
-
- Grollman A., Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet.: TIG. 1993;9:246–249. - PubMed
-
- Gannett P., Sura T. Base pairing of 8-oxoguanosine and 8-oxo-2′-deoxyguanosine with 2′-deoxyadenosine, 2′-deoxycytosine, 2′-deoxyguanosine, and thymidine. Chem. Res. Toxicol. 1993;6:690–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

