Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke
- PMID: 24746419
- PMCID: PMC4016169
- DOI: 10.1016/j.neuron.2014.03.003
Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke
Abstract
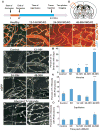
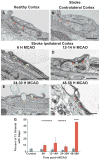
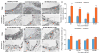
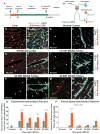
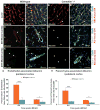
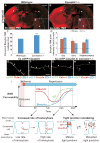
Brain endothelial cells form a paracellular and transcellular barrier to many blood-borne solutes via tight junctions (TJs) and scarce endocytotic vesicles. The blood-brain barrier (BBB) plays a pivotal role in the healthy and diseased CNS. BBB damage after ischemic stroke contributes to increased mortality, yet the contributions of paracellular and transcellular mechanisms to this process in vivo are unknown. We have created a transgenic mouse strain whose endothelial TJs are labeled with eGFP and have imaged dynamic TJ changes and fluorescent tracer leakage across the BBB in vivo, using two-photon microscopy in the t-MCAO stroke model. Although barrier function is impaired as early as 6 hr after stroke, TJs display profound structural defects only after 2 days. Conversely, the number of endothelial caveolae and transcytosis rate increase as early as 6 hr after stroke. Therefore, stepwise impairment of transcellular followed by paracellular barrier mechanisms accounts for the BBB deficits in stroke.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Caveolae-Mediated Endothelial Transcytosis across the Blood-Brain Barrier in Acute Ischemic Stroke.J Clin Med. 2021 Aug 25;10(17):3795. doi: 10.3390/jcm10173795. J Clin Med. 2021. PMID: 34501242 Free PMC article. Review.
-
Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke.Brain Pathol. 2022 Jan;32(1):e13006. doi: 10.1111/bpa.13006. Epub 2021 Jul 19. Brain Pathol. 2022. PMID: 34286899 Free PMC article.
-
Burns Impair Blood-Brain Barrier and Mesenchymal Stem Cells Can Reverse the Process in Mice.Front Immunol. 2020 Nov 6;11:578879. doi: 10.3389/fimmu.2020.578879. eCollection 2020. Front Immunol. 2020. PMID: 33240266 Free PMC article.
-
Storax Inhibits Caveolae-Mediated Transcytosis at Blood-Brain Barrier After Ischemic Stroke in Rats.Front Pharmacol. 2022 Jul 8;13:876235. doi: 10.3389/fphar.2022.876235. eCollection 2022. Front Pharmacol. 2022. PMID: 35873558 Free PMC article.
-
Blood-brain barrier dysfunction in ischemic stroke: _targeting tight junctions and transporters for vascular protection.Am J Physiol Cell Physiol. 2018 Sep 1;315(3):C343-C356. doi: 10.1152/ajpcell.00095.2018. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949404 Free PMC article. Review.
Cited by
-
Homotrimer cavin1 interacts with caveolin1 to facilitate tumor growth and activate microglia through extracellular vesicles in glioma.Theranostics. 2020 May 17;10(15):6674-6694. doi: 10.7150/thno.45688. eCollection 2020. Theranostics. 2020. PMID: 32550897 Free PMC article.
-
Vascular Sema3E-Plexin-D1 Signaling Reactivation Promotes Post-stroke Recovery through VEGF Downregulation in Mice.Transl Stroke Res. 2022 Feb;13(1):142-159. doi: 10.1007/s12975-021-00914-4. Epub 2021 May 12. Transl Stroke Res. 2022. PMID: 33978913 Free PMC article.
-
Dysfunction of Calcyphosine-Like gene impairs retinal angiogenesis through the MYC axis and is associated with familial exudative vitreoretinopathy.Elife. 2024 Sep 12;13:RP96907. doi: 10.7554/eLife.96907. Elife. 2024. PMID: 39264149 Free PMC article.
-
Blood-brain borders: a proposal to address limitations of historical blood-brain barrier terminology.Fluids Barriers CNS. 2024 Jan 5;21(1):3. doi: 10.1186/s12987-023-00478-5. Fluids Barriers CNS. 2024. PMID: 38183042 Free PMC article. Review.
-
Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage.Semin Neurol. 2016 Jun;36(3):288-97. doi: 10.1055/s-0036-1582132. Epub 2016 May 23. Semin Neurol. 2016. PMID: 27214704 Free PMC article. Review.
References
-
- Abbott N, Patabendige A, Dolman D, Yusof S, Begley D. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37:13–25. - PubMed
-
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13287–13292. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

