Epigenetic reprogramming induces the expansion of cord blood stem cells
- PMID: 24762436
- PMCID: PMC4038563
- DOI: 10.1172/JCI70313
Epigenetic reprogramming induces the expansion of cord blood stem cells
Abstract
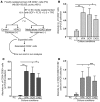
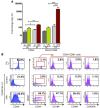
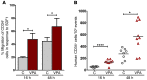
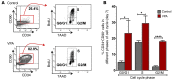
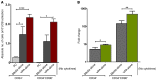
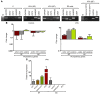
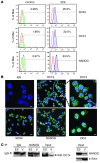
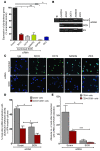
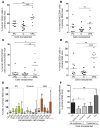
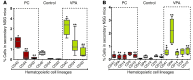
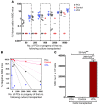
Cord blood (CB) cells that express CD34 have extensive hematopoietic capacity and rapidly divide ex vivo in the presence of cytokine combinations; however, many of these CB CD34+ cells lose their marrow-repopulating potential. To overcome this decline in function, we treated dividing CB CD34+ cells ex vivo with several histone deacetylase inhibitors (HDACIs). Treatment of CB CD34+ cells with the most active HDACI, valproic acid (VPA), following an initial 16-hour cytokine priming, increased the number of multipotent cells (CD34+CD90+) generated; however, the degree of expansion was substantially greater in the presence of both VPA and cytokines for a full 7 days. Treated CD34+ cells were characterized based on the upregulation of pluripotency genes, increased aldehyde dehydrogenase activity, and enhanced expression of CD90, c-Kit (CD117), integrin α6 (CD49f), and CXCR4 (CD184). Furthermore, siRNA-mediated inhibition of pluripotency gene expression reduced the generation of CD34+CD90+ cells by 89%. Compared with CB CD34+ cells, VPA-treated CD34+ cells produced a greater number of SCID-repopulating cells and established multilineage hematopoiesis in primary and secondary immune-deficient recipient mice. These data indicate that dividing CB CD34+ cells can be epigenetically reprogrammed by treatment with VPA so as to generate greater numbers of functional CB stem cells for use as transplantation grafts.
Figures


Comment in
-
Inhibiting HDAC for human hematopoietic stem cell expansion.J Clin Invest. 2014 Jun;124(6):2365-8. doi: 10.1172/JCI75803. Epub 2014 Apr 24. J Clin Invest. 2014. PMID: 24892711 Free PMC article.
Similar articles
-
Epigenetic and molecular signatures of cord blood CD34(+) cells treated with histone deacetylase inhibitors.Vox Sang. 2016 Jan;110(1):79-89. doi: 10.1111/vox.12303. Epub 2015 Jun 17. Vox Sang. 2016. PMID: 26084882
-
Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor.Stem Cells Transl Med. 2020 Apr;9(4):531-542. doi: 10.1002/sctm.19-0199. Epub 2020 Jan 17. Stem Cells Transl Med. 2020. PMID: 31950644 Free PMC article.
-
Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells.Biol Blood Marrow Transplant. 2014 Apr;20(4):480-9. doi: 10.1016/j.bbmt.2013.12.562. Epub 2013 Dec 26. Biol Blood Marrow Transplant. 2014. PMID: 24374212 Free PMC article.
-
Ex Vivo Expansion of CD34+ CD90+ CD49f+ Hematopoietic Stem and Progenitor Cells from Non-Enriched Umbilical Cord Blood with Azole Compounds.Stem Cells Transl Med. 2018 May;7(5):376-393. doi: 10.1002/sctm.17-0251. Epub 2018 Feb 2. Stem Cells Transl Med. 2018. PMID: 29392885 Free PMC article.
-
Role of epigenetic reprogramming in hematopoietic stem cell function.Curr Opin Hematol. 2015 Jul;22(4):279-85. doi: 10.1097/MOH.0000000000000143. Curr Opin Hematol. 2015. PMID: 26049747 Review.
Cited by
-
Valproic acid enhances neuronal differentiation of sympathoadrenal progenitor cells.Mol Psychiatry. 2015 Aug;20(8):941-50. doi: 10.1038/mp.2015.3. Epub 2015 Feb 24. Mol Psychiatry. 2015. PMID: 25707399
-
Effect of Small Molecule on ex vivo Expansion of Cord Blood Hematopoietic Stem Cells: A Concise Review.Front Cell Dev Biol. 2021 Apr 9;9:649115. doi: 10.3389/fcell.2021.649115. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898442 Free PMC article. Review.
-
Valproic Acid Decreases Endothelial Colony Forming Cells Differentiation and Induces Endothelial-to-Mesenchymal Transition-like Process.Stem Cell Rev Rep. 2020 Apr;16(2):357-368. doi: 10.1007/s12015-019-09950-y. Stem Cell Rev Rep. 2020. PMID: 31898801
-
DNA barcode to trace the development and differentiation of cord blood stem cells (Review).Mol Med Rep. 2021 Dec;24(6):849. doi: 10.3892/mmr.2021.12489. Epub 2021 Oct 13. Mol Med Rep. 2021. PMID: 34643250 Free PMC article. Review.
-
Ex Vivo Expansion of Hematopoietic Stem Cells for Therapeutic Purposes: Lessons from Development and the Niche.Cells. 2019 Feb 18;8(2):169. doi: 10.3390/cells8020169. Cells. 2019. PMID: 30781676 Free PMC article. Review.
References
-
- Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90(12):4665–4678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

