Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration
- PMID: 24926024
- PMCID: PMC4523269
- DOI: 10.1126/science.1242281
Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration
Abstract
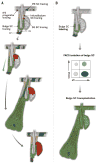
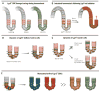
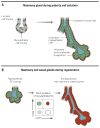
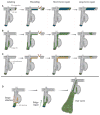
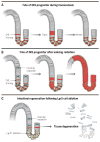
Tissues rely upon stem cells for homeostasis and repair. Recent studies show that the fate and multilineage potential of epithelial stem cells can change depending on whether a stem cell exists within its resident niche and responds to normal tissue homeostasis, whether it is mobilized to repair a wound, or whether it is taken from its niche and challenged to de novo tissue morphogenesis after transplantation. In this Review, we discuss how different populations of naturally lineage-restricted stem cells and committed progenitors can display remarkable plasticity and reversibility and reacquire long-term self-renewing capacities and multilineage differentiation potential during physiological and regenerative conditions. We also discuss the implications of cellular plasticity for regenerative medicine and for cancer.
Copyright © 2014, American Association for the Advancement of Science.
Figures


Similar articles
-
Harnessing the potential of lung stem cells for regenerative medicine.Int J Biochem Cell Biol. 2014 Nov;56:82-91. doi: 10.1016/j.biocel.2014.10.012. Epub 2014 Oct 15. Int J Biochem Cell Biol. 2014. PMID: 25450456 Review.
-
Cycling progenitors maintain epithelia while diverse cell types contribute to repair.Bioessays. 2013 May;35(5):443-51. doi: 10.1002/bies.201200166. Epub 2013 Mar 6. Bioessays. 2013. PMID: 23463676 Review.
-
The potential of mesothelial cells in tissue engineering and regenerative medicine applications.Int J Artif Organs. 2007 Jun;30(6):527-40. doi: 10.1177/039139880703000611. Int J Artif Organs. 2007. PMID: 17628854 Review.
-
Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche.Dev Cell. 2017 Nov 20;43(4):387-401. doi: 10.1016/j.devcel.2017.10.001. Dev Cell. 2017. PMID: 29161590 Free PMC article. Review.
-
Cellular mechanisms of epithelial stem cell self-renewal and differentiation during homeostasis and repair.Wiley Interdiscip Rev Dev Biol. 2020 Jan;9(1):e361. doi: 10.1002/wdev.361. Epub 2019 Aug 29. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31468728 Review.
Cited by
-
MicroRNAs Enhance Keratinocyte Proliferative Capacity in a Stem Cell-Enriched Epithelium.PLoS One. 2015 Aug 6;10(8):e0134853. doi: 10.1371/journal.pone.0134853. eCollection 2015. PLoS One. 2015. PMID: 26248284 Free PMC article.
-
Basal Progenitors Contribute to Repair of the Prostate Epithelium Following Induced Luminal Anoikis.Stem Cell Reports. 2016 May 10;6(5):660-667. doi: 10.1016/j.stemcr.2016.03.007. Epub 2016 Apr 21. Stem Cell Reports. 2016. PMID: 27117783 Free PMC article.
-
Cellular mechanisms underlying adult tissue plasticity in Drosophila.Fly (Austin). 2022 Dec;16(1):190-206. doi: 10.1080/19336934.2022.2066952. Fly (Austin). 2022. PMID: 35470772 Free PMC article. Review.
-
Cancer Stem Cells are Actually Stem Cells with Disordered Differentiation: the Monophyletic Origin of Cancer.Stem Cell Rev Rep. 2023 May;19(4):827-838. doi: 10.1007/s12015-023-10508-2. Epub 2023 Jan 17. Stem Cell Rev Rep. 2023. PMID: 36648606 Free PMC article. Review.
-
Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration.Nat Cell Biol. 2019 Nov;21(11):1321-1333. doi: 10.1038/s41556-019-0402-6. Epub 2019 Nov 4. Nat Cell Biol. 2019. PMID: 31685987 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

