Mechanisms underlying mutational signatures in human cancers
- PMID: 24981601
- PMCID: PMC6044419
- DOI: 10.1038/nrg3729
Mechanisms underlying mutational signatures in human cancers
Abstract
The collective somatic mutations observed in a cancer are the outcome of multiple mutagenic processes that have been operative over the lifetime of a patient. Each process leaves a characteristic imprint--a mutational signature--on the cancer genome, which is defined by the type of DNA damage and DNA repair processes that result in base substitutions, insertions and deletions or structural variations. With the advent of whole-genome sequencing, researchers are identifying an increasing array of these signatures. Mutational signatures can be used as a physiological readout of the biological history of a cancer and also have potential use for discerning ongoing mutational processes from historical ones, thus possibly revealing new _targets for anticancer therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures


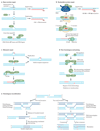

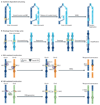
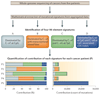

Comment in
-
Cancer genomics: A catalogue of somatic mutations.Nat Rev Genet. 2016 Jul;17(7):378. doi: 10.1038/nrg.2016.65. Epub 2016 May 9. Nat Rev Genet. 2016. PMID: 27156977 No abstract available.
Similar articles
-
Mutational spectra and mutational signatures: Insights into cancer aetiology and mechanisms of DNA damage and repair.DNA Repair (Amst). 2018 Nov;71:6-11. doi: 10.1016/j.dnarep.2018.08.003. Epub 2018 Aug 24. DNA Repair (Amst). 2018. PMID: 30236628 Free PMC article. Review.
-
MutationalPatterns: the one stop shop for the analysis of mutational processes.BMC Genomics. 2022 Feb 15;23(1):134. doi: 10.1186/s12864-022-08357-3. BMC Genomics. 2022. PMID: 35168570 Free PMC article.
-
Deciphering signatures of mutational processes operative in human cancer.Cell Rep. 2013 Jan 31;3(1):246-59. doi: 10.1016/j.celrep.2012.12.008. Epub 2013 Jan 10. Cell Rep. 2013. PMID: 23318258 Free PMC article.
-
Computational approaches for discovery of mutational signatures in cancer.Brief Bioinform. 2019 Jan 18;20(1):77-88. doi: 10.1093/bib/bbx082. Brief Bioinform. 2019. PMID: 28968631 Free PMC article. Review.
-
The repertoire of mutational signatures in human cancer.Nature. 2020 Feb;578(7793):94-101. doi: 10.1038/s41586-020-1943-3. Epub 2020 Feb 5. Nature. 2020. PMID: 32025018 Free PMC article.
Cited by
-
The topography of mutational processes in breast cancer genomes.Nat Commun. 2016 May 2;7:11383. doi: 10.1038/ncomms11383. Nat Commun. 2016. PMID: 27136393 Free PMC article.
-
A novel genomic classification system of gastric cancer via integrating multidimensional genomic characteristics.Gastric Cancer. 2021 Nov;24(6):1227-1241. doi: 10.1007/s10120-021-01201-9. Epub 2021 Jun 6. Gastric Cancer. 2021. PMID: 34095982 Free PMC article.
-
Systems medicine in colorectal cancer: from a mathematical model toward a new type of clinical trial.Wiley Interdiscip Rev Syst Biol Med. 2016 Jul;8(4):314-36. doi: 10.1002/wsbm.1342. Epub 2016 May 30. Wiley Interdiscip Rev Syst Biol Med. 2016. PMID: 27240214 Free PMC article. Review.
-
TP53 mutation status and consensus molecular subtypes of colorectal cancer in patients from Rwanda.BMC Cancer. 2024 Oct 11;24(1):1266. doi: 10.1186/s12885-024-13009-8. BMC Cancer. 2024. PMID: 39394554 Free PMC article.
-
EvoLSTM: context-dependent models of sequence evolution using a sequence-to-sequence LSTM.Bioinformatics. 2020 Jul 1;36(Suppl_1):i353-i361. doi: 10.1093/bioinformatics/btaa447. Bioinformatics. 2020. PMID: 32657367 Free PMC article.
References
-
- Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. [This study shows the prevalence of somatic mutations in human cancer genomes, which indicates that most of the mutations do not drive oncogenesis. Nevertheless, it provides evidence for driver mutations that are actively involved in tumour development.] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

