The potential role of GLUT4 transporters and insulin receptors in the hypoglycaemic activity of Ficus lutea acetone leaf extract
- PMID: 25070239
- PMCID: PMC4137081
- DOI: 10.1186/1472-6882-14-269
The potential role of GLUT4 transporters and insulin receptors in the hypoglycaemic activity of Ficus lutea acetone leaf extract
Abstract
Background: Some Ficus species have been used in traditional African medicine in the treatment of diabetes. The antidiabetic potential of certain species has been confirmed in vivo but the mechanism of activity remains uncertain. The aim was to investigate the hypoglycaemic potential of ten Ficus species focussing on glucose uptake, insulin secretion and the possible mechanism of hypoglycaemic activity.
Methods: The dried and ground leaves of ten Ficus species were extracted with acetone. The dried acetone extract was reconstituted with DMSO to a concentration of 100 mg/ml which was then serially diluted and used to assay for glucose uptake in muscle, fat and liver cells, and insulin secretion in pancreatic cells.
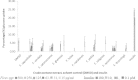
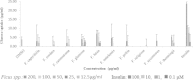
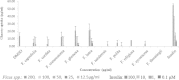
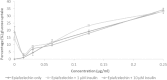
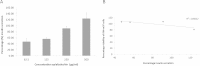
Results: Only the F. lutea extract was able to modulate glucose metabolism. In comparison to insulin in the primary muscle cells, the glucose uptake ability of the extract was 33% as effective. In the hepatoma cell line, the extract was as effective as metformin in decreasing extracellular glucose concentration by approximately 20%. In the pancreatic insulin secretory assay, the extract was 4 times greater in its secretory activity than commercial glibenclamide. With F. lutea extract significantly increasing glucose uptake in the primary muscle cells, primary fat cells, C2C12 muscle and H-4-II-E liver cells, the extract may act by increasing the activity of cell surface glucose transporters. When the 3T3-L1 pre-adipocytes were compared to the primary muscle, primary fat and C2C12 cells, the differences in the former's ability to transport glucose into the cell may be due to the absence of the GLUT4 transporter, which on activation via the insulin receptor decreases extracellular glucose concentrations. Because the pre-adipocytes failed to show any active increase in glucose uptake, the present effect has to be linked to the absence of the GLUT4 transporter.
Conclusion: Only F. lutea possessed substantial in vitro activity related to glucose metabolism. Based on the effect produced in the various cell types, F. lutea also appears to be a partial agonist/antagonist of the insulin cell membrane receptor. While the clinical effectiveness of F. lutea is not known, this plant species does possess the ability to modify glucose metabolism.
Figures
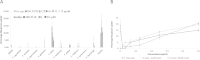




Similar articles
-
Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: comparison of an in vitro assay with an in vivo obese mouse model.BMC Complement Altern Med. 2016 Mar 31;16:110. doi: 10.1186/s12906-016-1087-z. BMC Complement Altern Med. 2016. PMID: 27029351 Free PMC article.
-
Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes.BMC Complement Altern Med. 2013 May 4;13:94. doi: 10.1186/1472-6882-13-94. BMC Complement Altern Med. 2013. PMID: 23641947 Free PMC article.
-
Defatting of acetone leaf extract of Acacia karroo (Hayne) enhances its hypoglycaemic potential.BMC Complement Altern Med. 2017 Oct 23;17(1):482. doi: 10.1186/s12906-017-1987-6. BMC Complement Altern Med. 2017. PMID: 29058615 Free PMC article.
-
Evaluation of hypoglycemic activity and toxicity profiles of the leaves of Ficus deltoidea in rodents.J Complement Integr Med. 2011 Jan;8. doi: 10.2202/1553-3840.1469. J Complement Integr Med. 2011. PMID: 22754938
-
A Citrullus colocynthis fruit extract acutely enhances insulin-induced GLUT4 translocation and glucose uptake in adipocytes by increasing PKB phosphorylation.J Ethnopharmacol. 2021 Apr 24;270:113772. doi: 10.1016/j.jep.2020.113772. Epub 2021 Jan 6. J Ethnopharmacol. 2021. PMID: 33418030
Cited by
-
Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract.Molecules. 2023 Nov 22;28(23):7717. doi: 10.3390/molecules28237717. Molecules. 2023. PMID: 38067448 Free PMC article.
-
Antidiabetic Potential of Medicinal Plants and Their Active Components.Biomolecules. 2019 Sep 30;9(10):551. doi: 10.3390/biom9100551. Biomolecules. 2019. PMID: 31575072 Free PMC article. Review.
-
Annona stenophylla aqueous extract stimulate glucose uptake in established C2Cl2 muscle cell lines.Afr Health Sci. 2019 Jun;19(2):2219-2229. doi: 10.4314/ahs.v19i2.47. Afr Health Sci. 2019. PMID: 31656507 Free PMC article.
-
Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: Possible role in prevention of diabetic complications.J Tradit Complement Med. 2016 Mar 2;7(1):54-64. doi: 10.1016/j.jtcme.2016.01.002. eCollection 2017 Jan. J Tradit Complement Med. 2016. PMID: 28053889 Free PMC article.
-
Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: comparison of an in vitro assay with an in vivo obese mouse model.BMC Complement Altern Med. 2016 Mar 31;16:110. doi: 10.1186/s12906-016-1087-z. BMC Complement Altern Med. 2016. PMID: 27029351 Free PMC article.
References
-
- Kamboj VP. Herbal medicine. Curr Sci-Bangalore. 2000;78(1):35–38.
-
- Cousins D, Huffman MA. Medicinal properties in the diet of gorillas: An ethno- pharmacological evaluation. Afr Stud Monogr. 2002;23:65–89.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1472-6882/14/269/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

