WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic instability and carcinogenesis
- PMID: 25088202
- PMCID: PMC5547996
- DOI: 10.1038/onc.2014.239
WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic instability and carcinogenesis
Abstract
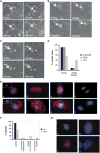
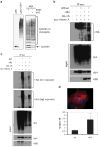
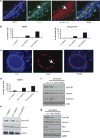
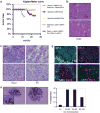
The tyrosine kinase WEE1 controls the timing of entry into mitosis in eukaryotes and its genetic deletion leads to pre-implantation lethality in mice. Here, we show that besides the premature mitotic entry phenotype, Wee1 mutant murine cells fail to complete mitosis properly and exhibit several additional defects that contribute to the deregulation of mitosis, allowing mutant cells to progress through mitosis at the expense of genomic integrity. WEE1 interacts with the anaphase promoting complex, functioning as a negative regulator, and the deletion of Wee1 results in hyper-activation of this complex. Mammary specific knockout mice overcome the DNA damage response pathway triggered by the mis-coordination of the cell cycle in mammary epithelial cells and heterozygote mice spontaneously develop mammary tumors. Thus, WEE1 functions as a haploinsufficient tumor suppressor that coordinates distinct cell division events to allow correct segregation of genetic information into daughter cells and maintain genome integrity.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures



Similar articles
-
Expression of constitutively active CDK1 stabilizes APC-Cdh1 substrates and potentiates premature spindle assembly and checkpoint function in G1 cells.PLoS One. 2012;7(3):e33835. doi: 10.1371/journal.pone.0033835. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479455 Free PMC article.
-
Murine Wee1 plays a critical role in cell cycle regulation and pre-implantation stages of embryonic development.Int J Biol Sci. 2006;2(4):161-70. doi: 10.7150/ijbs.2.161. Epub 2006 May 18. Int J Biol Sci. 2006. PMID: 16810330 Free PMC article.
-
Redundant ubiquitin ligase activities regulate wee1 degradation and mitotic entry.Cell Cycle. 2007 Nov 15;6(22):2795-9. doi: 10.4161/cc.6.22.4919. Epub 2007 Aug 20. Cell Cycle. 2007. PMID: 18032919
-
Tome-1, wee1, and the onset of mitosis: coupled destruction for timely entry.Mol Cell. 2003 Apr;11(4):845-6. doi: 10.1016/s1097-2765(03)00149-7. Mol Cell. 2003. PMID: 12718868 Review.
-
Triggering mitosis.FEBS Lett. 2019 Oct;593(20):2868-2888. doi: 10.1002/1873-3468.13635. Epub 2019 Oct 24. FEBS Lett. 2019. PMID: 31602636 Review.
Cited by
-
Growth-Dependent Activation of Protein Kinases Suggests a Mechanism for Measuring Cell Growth.Genetics. 2020 Jul;215(3):729-746. doi: 10.1534/genetics.120.303200. Epub 2020 May 27. Genetics. 2020. PMID: 32461268 Free PMC article.
-
A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic _target.J Hematol Oncol. 2020 Sep 21;13(1):126. doi: 10.1186/s13045-020-00959-2. J Hematol Oncol. 2020. PMID: 32958072 Free PMC article. Review.
-
WEE1 inhibition _targets cell cycle checkpoints for triple negative breast cancers to overcome cisplatin resistance.Sci Rep. 2017 Mar 6;7:43517. doi: 10.1038/srep43517. Sci Rep. 2017. PMID: 28262781 Free PMC article.
-
METTL3 Regulates Liver Homeostasis, Hepatocyte Ploidy, and Circadian Rhythm-Controlled Gene Expression in Mice.Am J Pathol. 2022 Jan;192(1):56-71. doi: 10.1016/j.ajpath.2021.09.005. Epub 2021 Sep 29. Am J Pathol. 2022. PMID: 34599880 Free PMC article.
-
Loss of the puromycin-sensitive aminopeptidase, PAM-1, triggers the spindle assembly checkpoint during the first mitotic division in Caenorhabditis elegans.MicroPubl Biol. 2024 Apr 2;2024:10.17912/micropub.biology.001167. doi: 10.17912/micropub.biology.001167. eCollection 2024. MicroPubl Biol. 2024. PMID: 38633870 Free PMC article.
References
-
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. - PubMed
-
- Thuriaux P, Nurse P, Carter B. Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1978;161:215–220. - PubMed
-
- Fantes PA, Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978;115:317–329. - PubMed
-
- Squire CJ, Dickson JM, Ivanovic I, Baker EN. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure. 2005;13:541–550. - PubMed
-
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

