The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men
- PMID: 25168616
- PMCID: PMC4407822
- DOI: 10.1016/j.eururo.2014.08.011
The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men
Abstract
Background: Prostate-specific antigen (PSA) testing has limited accuracy for the early detection of prostate cancer (PCa).
Objective: To assess the value added by percentage of free to total PSA (%fPSA), prostate cancer antigen 3 (PCA3), and a kallikrein panel (4k-panel) to the European Randomised Study of Screening for Prostate Cancer (ERSPC) multivariable prediction models: risk calculator (RC) 4, including transrectal ultrasound, and RC 4 plus digital rectal examination (4+DRE) for prescreened men.
Design, setting, and participants: Participants were invited for rescreening between October 2007 and February 2009 within the Dutch part of the ERSPC study. Biopsies were taken in men with a PSA level ≥3.0 ng/ml or a PCA3 score ≥10. Additional analyses of the 4k-panel were done on serum samples.
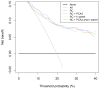
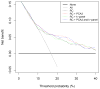
Outcome measurements and statistical analysis: Outcome was defined as PCa detectable by sextant biopsy. Receiver operating characteristic curve and decision curve analyses were performed to compare the predictive capabilities of %fPSA, PCA3, 4k-panel, the ERSPC RCs, and their combinations in logistic regression models.
Results and limitations: PCa was detected in 119 of 708 men. The %fPSA did not perform better univariately or added to the RCs compared with the RCs alone. In 202 men with an elevated PSA, the 4k-panel discriminated better than PCA3 when modelled univariately (area under the curve [AUC]: 0.78 vs. 0.62; p=0.01). The multivariable models with PCA3 or the 4k-panel were equivalent (AUC: 0.80 for RC 4+DRE). In the total population, PCA3 discriminated better than the 4k-panel (univariate AUC: 0.63 vs. 0.56; p=0.05). There was no statistically significant difference between the multivariable model with PCA3 (AUC: 0.73) versus the model with the 4k-panel (AUC: 0.71; p=0.18). The multivariable model with PCA3 performed better than the reference model (0.73 vs. 0.70; p=0.02). Decision curves confirmed these patterns, although numbers were small.
Conclusions: Both PCA3 and, to a lesser extent, a 4k-panel have added value to the DRE-based ERSPC RC in detecting PCa in prescreened men.
Patient summary: We studied the added value of novel biomarkers to previously developed risk prediction models for prostate cancer. We found that inclusion of these biomarkers resulted in an increase in predictive ability.
Keywords: Kallikrein panel (4k-panel); Percentage of free to total PSA; Prostate biopsy; Prostate cancer; Prostate cancer antigen 3 (PCA3); Prostate cancer risk calculator; Validation.
Copyright © 2014. Published by Elsevier B.V.
Figures
Comment in
-
Re: The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men.J Urol. 2015 Jan;193(1):127. doi: 10.1016/j.juro.2014.10.074. Epub 2014 Oct 22. J Urol. 2015. PMID: 25523657 No abstract available.
Similar articles
-
Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators.Eur Urol. 2012 Mar;61(3):577-83. doi: 10.1016/j.eururo.2011.11.012. Epub 2011 Nov 15. Eur Urol. 2012. PMID: 22104592
-
Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment.Eur Urol. 2016 Jul;70(1):45-53. doi: 10.1016/j.eururo.2015.04.039. Epub 2015 May 16. Eur Urol. 2016. PMID: 25985884 Free PMC article.
-
Re: The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men.J Urol. 2015 Jan;193(1):127. doi: 10.1016/j.juro.2014.10.074. Epub 2014 Oct 22. J Urol. 2015. PMID: 25523657 No abstract available.
-
Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis.Ann Oncol. 2015 May;26(5):848-864. doi: 10.1093/annonc/mdu525. Epub 2014 Nov 17. Ann Oncol. 2015. PMID: 25403590 Review.
-
Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions.Prostate Cancer Prostatic Dis. 2017 Mar;20(1):12-19. doi: 10.1038/pcan.2016.59. Epub 2016 Dec 6. Prostate Cancer Prostatic Dis. 2017. PMID: 27922627 Review.
Cited by
-
Guidance on patient consultation. Current evidence for prostate-specific antigen screening in healthy men and treatment options for men with proven localised prostate cancer.Curr Urol Rep. 2015 May;16(5):28. doi: 10.1007/s11934-015-0502-1. Curr Urol Rep. 2015. PMID: 25773347 Review.
-
Prostate-based biofluids for the detection of prostate cancer: A comparative study of the diagnostic performance of cell-sourced RNA biomarkers.Prostate Int. 2016 Sep;4(3):97-102. doi: 10.1016/j.prnil.2016.04.002. Epub 2016 May 5. Prostate Int. 2016. PMID: 27689066 Free PMC article.
-
Diagnostic and prognostic factors in patients with prostate cancer: a systematic review.BMJ Open. 2022 Apr 4;12(4):e058267. doi: 10.1136/bmjopen-2021-058267. BMJ Open. 2022. PMID: 35379637 Free PMC article.
-
Development and validation of a nomogram including lymphocyte-to-monocyte ratio for initial prostate biopsy: a double-center retrospective study.Asian J Androl. 2021 Jan-Feb;23(1):41-46. doi: 10.4103/aja.aja_19_20. Asian J Androl. 2021. PMID: 32503957 Free PMC article.
-
Developing a model for forecasting Gleason score ≥7 in potential prostate cancer patients to reduce unnecessary prostate biopsies.Int Urol Nephrol. 2016 Apr;48(4):535-40. doi: 10.1007/s11255-016-1218-y. Epub 2016 Jan 25. Int Urol Nephrol. 2016. PMID: 26810323
References
-
- Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. - PubMed
-
- Draisma G, Boer R, Otto SJ, van der Cruijsen IW, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. - PubMed
-
- Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schroder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–12. discussion 112. - PubMed
-
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

