Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-_targeting mammalian _target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase
- PMID: 25212826
- PMCID: PMC4303114
- DOI: 10.1186/s13058-014-0430-x
Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-_targeting mammalian _target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase
Abstract
Introduction: Activation of the phosphatidylinositol 3-kinase (PI3K) pathway in estrogen receptor α (ER)-positive breast cancer is associated with reduced ER expression and activity, luminal B subtype, and poor outcome. Phosphatase and tensin homolog (PTEN), a negative regulator of this pathway, is typically lost in ER-negative breast cancer. We set out to clarify the role of reduced PTEN levels in endocrine resistance, and to explore the combination of newly developed PI3K downstream kinase inhibitors to overcome this resistance.
Methods: Altered cellular signaling, gene expression, and endocrine sensitivity were determined in inducible PTEN-knockdown ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer cell and/or xenograft models. Single or two-agent combinations of kinase inhibitors were examined to improve endocrine therapy.
Results: Moderate PTEN reduction was sufficient to enhance PI3K signaling, generate a gene signature associated with the luminal B subtype of breast cancer, and cause endocrine resistance in vitro and in vivo. The mammalian _target of rapamycin (mTOR), protein kinase B (AKT), or mitogen-activated protein kinase kinase (MEK) inhibitors, alone or in combination, improved endocrine therapy, but the efficacy varied by PTEN levels, type of endocrine therapy, and the specific inhibitor(s). A single-agent AKT inhibitor combined with fulvestrant conferred superior efficacy in overcoming resistance, inducing apoptosis and tumor regression.
Conclusions: Moderate reduction in PTEN, without complete loss, can activate the PI3K pathway to cause endocrine resistance in ER-positive breast cancer, which can be overcome by combining endocrine therapy with inhibitors of the PI3K pathway. Our data suggests that the ER degrader fulvestrant, to block both ligand-dependent and -independent ER signaling, combined with an AKT inhibitor is an effective strategy to test in patients.
Figures
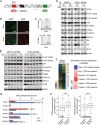
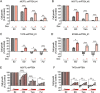
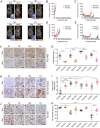


Comment in
-
_targeting PTEN-defined breast cancers with a one-two punch.Breast Cancer Res. 2015 Apr 8;17(1):51. doi: 10.1186/s13058-015-0566-3. Breast Cancer Res. 2015. PMID: 25888162 Free PMC article.
Similar articles
-
Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer.Breast Cancer Res. 2011 Mar 1;13(2):R21. doi: 10.1186/bcr2833. Breast Cancer Res. 2011. PMID: 21362200 Free PMC article.
-
Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro.Breast Cancer Res. 2014 Jan 23;16(1):R12. doi: 10.1186/bcr3604. Breast Cancer Res. 2014. PMID: 24457069 Free PMC article.
-
AKT Antagonist AZD5363 Influences Estrogen Receptor Function in Endocrine-Resistant Breast Cancer and Synergizes with Fulvestrant (ICI182780) In Vivo.Mol Cancer Ther. 2015 Sep;14(9):2035-48. doi: 10.1158/1535-7163.MCT-15-0143. Epub 2015 Jun 26. Mol Cancer Ther. 2015. PMID: 26116361
-
Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer.Breast Cancer. 2018 Jul;25(4):392-401. doi: 10.1007/s12282-017-0812-x. Epub 2017 Oct 31. Breast Cancer. 2018. PMID: 29086897 Review.
-
_targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.Cancer Treat Rev. 2014 Aug;40(7):862-71. doi: 10.1016/j.ctrv.2014.03.004. Epub 2014 Mar 26. Cancer Treat Rev. 2014. PMID: 24774538 Review.
Cited by
-
Selective AKT kinase inhibitor capivasertib in combination with fulvestrant in PTEN-mutant ER-positive metastatic breast cancer.NPJ Breast Cancer. 2021 Apr 16;7(1):44. doi: 10.1038/s41523-021-00251-7. NPJ Breast Cancer. 2021. PMID: 33863913 Free PMC article.
-
PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around.Cancers (Basel). 2019 Aug 9;11(8):1141. doi: 10.3390/cancers11081141. Cancers (Basel). 2019. PMID: 31404976 Free PMC article. Review.
-
Overview of the therapeutic strategies for ER positive breast cancer.Biochem Pharmacol. 2023 Jun;212:115552. doi: 10.1016/j.bcp.2023.115552. Epub 2023 Apr 15. Biochem Pharmacol. 2023. PMID: 37068524 Free PMC article. Review.
-
Challenges in the management of advanced, ER-positive, HER2-negative breast cancer.Nat Rev Clin Oncol. 2015 Sep;12(9):541-52. doi: 10.1038/nrclinonc.2015.99. Epub 2015 May 26. Nat Rev Clin Oncol. 2015. PMID: 26011489 Review.
-
Association of Abl interactor 2, ABI2, with platelet/lymphocyte ratio in patients with renal cell carcinoma: A pilot study.Int J Exp Pathol. 2020 Jun;101(3-4):87-95. doi: 10.1111/iep.12349. Epub 2020 Jun 4. Int J Exp Pathol. 2020. PMID: 32496656 Free PMC article.
References
-
- Rimawi MF, Wiechmann LS, Wang YC, Huang C, Migliaccio I, Wu MF, Gutierrez C, Hilsenbeck SG, Arpino G, Massarweh S, Ward R, Soliz R, Osborne CK, Schiff R. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res. 2011;17:1351–1361. doi: 10.1158/1078-0432.CCR-10-1905. - DOI - PMC - PubMed
-
- Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, Lluch A, Gray JW, Brown PH, Hilsenbeck SG, Osborne CK, Mills GB, Lee AV, Schiff R: Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER + breast cancer.Breast Cancer Res 2010, 12:R40., - PMC - PubMed
-
- Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D, Gonzalez-Angulo AM, Pusztai L, Symmans WF, Bardelli A, Ellis P, Tutt AN, Gillett CE, Hennessy BT, Mills GB, Phillips WA, Piccart MJ, Speed TP, McArthur GA, Sotiriou C. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci U S A. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA94118/CA/NCI NIH HHS/United States
- AI036211/AI/NIAID NIH HHS/United States
- S10RR024574/RR/NCRR NIH HHS/United States
- P30CA125123/CA/NCI NIH HHS/United States
- R01 CA151962/CA/NCI NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- P50 CA058183/CA/NCI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- R01 CA094118/CA/NCI NIH HHS/United States
- P50 CA58183/CA/NCI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P50 CA58207/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

